For pdf-files of publications, please ask here or request login for members area. Thanks.
Most publications are also accessible from the website of
- 2026
- 2025
- 2024
- 2023
- 2022
- 2021
- 2020
- 2019
- 2018
- 2017
- 2016
- 2015
- 2014
- Book chapt.
- Patents
- Phd th.
Creep Resistant and Reprocessable Polyamide Networks Based on Reversible Lactone Ring-Opening
B. Daelman, J. Debuyck, V. Scholiers, J.M. Winne, F.E. Du Prez
Angew. Chem. Int. Ed., 2026
B. Daelman, J. Debuyck, V. Scholiers, J.M. Winne, F.E. Du Prez
Angew. Chem. Int. Ed., 2026
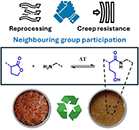 Abstract Amides are robust chemical linkages of interest in the field of dynamic covalent polymer networks (DCPNs), but their activation towards dynamic exchange remains an outstanding challenge. Herein, we introduce γ-hydroxy amides as a versatile motif for the design of creep resistant, yet fully reprocessable crosslinked polyamide-based materials. This was achieved by the reversible ring-opening of γ-lactones with primary amines. Small molecule kinetic studies showed that temperatures exceeding 120 °C are required for the ring-closure to occur at a significant rate. Thus, efficient exchange of the γ-hydroxy amide bonds was expected upon heating, while essentially non-dynamic covalent amide bonds should prevail at lower temperatures. γ-Hydroxy amides were then introduced into DCPNs, for which a newly prepared bifunctional γ-lactone monomer was cured with amine hardeners. A marked thermal response was observed in the rheological behavior, while creep resistance comparable to that of a non-dynamic epoxy-amine network was maintained up to 120 °C. Finally, we could also demonstrate the thermal resilience of γ-hydroxy amides after multiple compression molding cycles for a material with a glass transition temperature above 80 °C. Consequently, we expect that this simple chemistry platform has high potential for application in reprocessable thermoset materials.
Abstract Amides are robust chemical linkages of interest in the field of dynamic covalent polymer networks (DCPNs), but their activation towards dynamic exchange remains an outstanding challenge. Herein, we introduce γ-hydroxy amides as a versatile motif for the design of creep resistant, yet fully reprocessable crosslinked polyamide-based materials. This was achieved by the reversible ring-opening of γ-lactones with primary amines. Small molecule kinetic studies showed that temperatures exceeding 120 °C are required for the ring-closure to occur at a significant rate. Thus, efficient exchange of the γ-hydroxy amide bonds was expected upon heating, while essentially non-dynamic covalent amide bonds should prevail at lower temperatures. γ-Hydroxy amides were then introduced into DCPNs, for which a newly prepared bifunctional γ-lactone monomer was cured with amine hardeners. A marked thermal response was observed in the rheological behavior, while creep resistance comparable to that of a non-dynamic epoxy-amine network was maintained up to 120 °C. Finally, we could also demonstrate the thermal resilience of γ-hydroxy amides after multiple compression molding cycles for a material with a glass transition temperature above 80 °C. Consequently, we expect that this simple chemistry platform has high potential for application in reprocessable thermoset materials.
Chemical Upcycling of Polybutadiene Into Polyolefin-Based Dynamic Covalent Polymer Networks
V. Scholiers, C. Vos, J.M. Winne, D. De Vos, F.E. Du Prez
Macromol. Rapid Commun., 2026
V. Scholiers, C. Vos, J.M. Winne, D. De Vos, F.E. Du Prez
Macromol. Rapid Commun., 2026
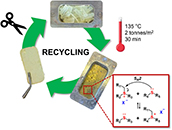 Abstract The use of non-renewable petrochemical feedstocks for the production of specialty polymer materials is not a sustainable practice. However, the use of waste commodity thermoplastic polymers as a feedstock for the production of specialty monomers is an attractive alternative. In this work, we show that high molar mass polybutadiene can be upcycled into reprocessable thermoset materials that can be recycled and reshaped multiple times as a dynamic covalent polymer network (DCPN). First, a one-pot partial hydrogenation and ethenolysis protocol was used to chemically cleave polybutadiene chains into low molecular weight α,ω-dienes. These were subsequently incorporated in a thiol-ene cured thermoset material using multifunctional thiols. The thioether linkages, connecting the polyolefin segments, can be turned into covalent dynamic moieties upon alkylation at elevated temperatures. The dynamic behavior of these thioether linkages was demonstrated through rheological and reprocessing experiments at elevated temperatures, highlighting their potential for sustainable material design. This strategy has a clear potential for an “upcycling” paradigm of bulk polymer waste into specialty polyolefin-based thermosets and DCPNs with appealing recyclability potential.
Abstract The use of non-renewable petrochemical feedstocks for the production of specialty polymer materials is not a sustainable practice. However, the use of waste commodity thermoplastic polymers as a feedstock for the production of specialty monomers is an attractive alternative. In this work, we show that high molar mass polybutadiene can be upcycled into reprocessable thermoset materials that can be recycled and reshaped multiple times as a dynamic covalent polymer network (DCPN). First, a one-pot partial hydrogenation and ethenolysis protocol was used to chemically cleave polybutadiene chains into low molecular weight α,ω-dienes. These were subsequently incorporated in a thiol-ene cured thermoset material using multifunctional thiols. The thioether linkages, connecting the polyolefin segments, can be turned into covalent dynamic moieties upon alkylation at elevated temperatures. The dynamic behavior of these thioether linkages was demonstrated through rheological and reprocessing experiments at elevated temperatures, highlighting their potential for sustainable material design. This strategy has a clear potential for an “upcycling” paradigm of bulk polymer waste into specialty polyolefin-based thermosets and DCPNs with appealing recyclability potential.
Monodisperse Oligoamides as Precise Precursors for Nylon via Solid-State Polycondensation
A. Tharayil, J. J.J. Verstappen, I. De Franceschi, K.V. Bernaerts, N. Badi, F.E. Du Prez
ACS Macro Lett., 2025
A. Tharayil, J. J.J. Verstappen, I. De Franceschi, K.V. Bernaerts, N. Badi, F.E. Du Prez
ACS Macro Lett., 2025
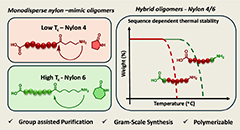 Abstract This study presents a scalable, solution-phase protocol for the synthesis of monodisperse oligoamides with nylon-4, nylon-6 and hybrid nylon-4/6 backbones, enabled by a group assisted purification strategy. For this, unnatural amino acid monomers are iteratively coupled onto a phosphonate-functional soluble support, yielding uniform oligomers bearing orthogonal functional end groups that allow their solid-state polycondensation (SSP). Pure monodisperse oligomer precursors of nylon-4 exhibits thermal limitations due to their low ceiling temperature, resulting in low molecular weight products after SSP. In contrast, incorporation of strategically positioned nylon-6 units effectively suppress backbiting and facilitates efficient SSP, producing well-defined nylon-4/6 copolymers. This unique design approach offers unprecedented control over the polyamide microstructure and establishes a versatile route toward polyamide synthesis with potential for customizing material properties.
Abstract This study presents a scalable, solution-phase protocol for the synthesis of monodisperse oligoamides with nylon-4, nylon-6 and hybrid nylon-4/6 backbones, enabled by a group assisted purification strategy. For this, unnatural amino acid monomers are iteratively coupled onto a phosphonate-functional soluble support, yielding uniform oligomers bearing orthogonal functional end groups that allow their solid-state polycondensation (SSP). Pure monodisperse oligomer precursors of nylon-4 exhibits thermal limitations due to their low ceiling temperature, resulting in low molecular weight products after SSP. In contrast, incorporation of strategically positioned nylon-6 units effectively suppress backbiting and facilitates efficient SSP, producing well-defined nylon-4/6 copolymers. This unique design approach offers unprecedented control over the polyamide microstructure and establishes a versatile route toward polyamide synthesis with potential for customizing material properties.
Dynamic β-Amino Sulfonamides for the Synthesis of Covalent Adaptable Networks
S. Maes, L.T. Nguyen, J.M. Winne, F.E. Du Prez
ACS Macro Lett., 2025
S. Maes, L.T. Nguyen, J.M. Winne, F.E. Du Prez
ACS Macro Lett., 2025
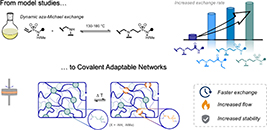 Abstract Thermosets are indispensable for their lightweight and excellent thermomechanical properties but suffer from nonrecyclability, exacerbating plastic pollution. Covalent Adaptable Networks (CANs), leveraging dynamic covalent bonds, address this challenge. This study explores β-amino sulfonamides (BASA) as novel dynamic and thermoreversible linkers for CANs. Compared to previously investigated β-amino amides (BAAs), small molecule BASA studies show a faster amine exchange reaction via (retro)aza-Michael reactions in a temperature window from 140 to 180 °C. More importantly, in contrast to the acrylamide monomers released from BAAs, the vinyl sulfonamide monomers released from BASAs are more robust and can resist homopolymerization at elevated temperatures. We showed that bisfunctional vinyl sulfonamide cross-linkers enable the synthesis of CANs with tunable properties, as verified through thermomechanical and rheological analysis. The anticipated increased thermal stability of BASA-based CANs was shown by the retention of mechanical and rheological performance over at least five recycling cycles.
Abstract Thermosets are indispensable for their lightweight and excellent thermomechanical properties but suffer from nonrecyclability, exacerbating plastic pollution. Covalent Adaptable Networks (CANs), leveraging dynamic covalent bonds, address this challenge. This study explores β-amino sulfonamides (BASA) as novel dynamic and thermoreversible linkers for CANs. Compared to previously investigated β-amino amides (BAAs), small molecule BASA studies show a faster amine exchange reaction via (retro)aza-Michael reactions in a temperature window from 140 to 180 °C. More importantly, in contrast to the acrylamide monomers released from BAAs, the vinyl sulfonamide monomers released from BASAs are more robust and can resist homopolymerization at elevated temperatures. We showed that bisfunctional vinyl sulfonamide cross-linkers enable the synthesis of CANs with tunable properties, as verified through thermomechanical and rheological analysis. The anticipated increased thermal stability of BASA-based CANs was shown by the retention of mechanical and rheological performance over at least five recycling cycles.
Turning down the heat: catalyst-free, low-temperature chemical degradation of thermoplastic polyurethanes
A. Roig, H. Wang, F.E. Du Prez
Polym. Chem., 2025
A. Roig, H. Wang, F.E. Du Prez
Polym. Chem., 2025
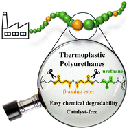 Abstract Thermoplastic polyurethanes (TPUs) constitute one of the most industrially relevant polymers because of their versatile and tunable properties, which makes them very interesting for a wide range of applications. However, their chemical degradability and recyclability is only achievable through hydrolysis or glycolysis of the urethane moieties, which typically occurs when using catalysts and elevated temperatures, thus resulting in a poorly cost- and energy-efficient process. Herein, we report a novel strategy where variable amounts of chemically degradable β-amino ester (BAE) moieties are incorporated into the main backbone of the thermoplastic urethanes. The amino group in the β-position of the ester was thought to act as an internal, covalently bonded catalyst that not only enables the preparation of materials without the use of external and potentially leachable toxic catalysts, but also the chemical degradation of the resulting TPUs under relatively mild conditions and shorter times via a straightforward transesterification process. The addition of different amounts of BAE groups has been investigated for both aliphatic and aromatic polyurethane thermoplastic backbones. Their chemical, structural and thermal properties have been investigated in-depth, and kinetic studies have been additionally performed with low molar mass model compounds to find the optimal conditions for their chemical degradation. The final aim of this bottom-up molecular design was not only to enable a catalyst-free polyurethane synthesis but also to open new avenues for developing TPUs with an enhanced, cost-effective and energy-efficient degradation process.
Abstract Thermoplastic polyurethanes (TPUs) constitute one of the most industrially relevant polymers because of their versatile and tunable properties, which makes them very interesting for a wide range of applications. However, their chemical degradability and recyclability is only achievable through hydrolysis or glycolysis of the urethane moieties, which typically occurs when using catalysts and elevated temperatures, thus resulting in a poorly cost- and energy-efficient process. Herein, we report a novel strategy where variable amounts of chemically degradable β-amino ester (BAE) moieties are incorporated into the main backbone of the thermoplastic urethanes. The amino group in the β-position of the ester was thought to act as an internal, covalently bonded catalyst that not only enables the preparation of materials without the use of external and potentially leachable toxic catalysts, but also the chemical degradation of the resulting TPUs under relatively mild conditions and shorter times via a straightforward transesterification process. The addition of different amounts of BAE groups has been investigated for both aliphatic and aromatic polyurethane thermoplastic backbones. Their chemical, structural and thermal properties have been investigated in-depth, and kinetic studies have been additionally performed with low molar mass model compounds to find the optimal conditions for their chemical degradation. The final aim of this bottom-up molecular design was not only to enable a catalyst-free polyurethane synthesis but also to open new avenues for developing TPUs with an enhanced, cost-effective and energy-efficient degradation process.
Exploring Transamidation and Chemical Recycling of β-Amino Amide-Derived Covalent Adaptable Networks
L.T. Nguyen, A.Tharayil, N.Badi, J.M. Winne, F.E. Du Prez
Polym. Chem., 2025
L.T. Nguyen, A.Tharayil, N.Badi, J.M. Winne, F.E. Du Prez
Polym. Chem., 2025
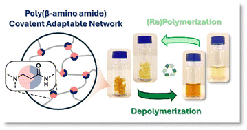 Abstract The growing environmental challenge of non-recyclable thermosets underscores the urgent need for sustainable alternatives. Covalent adaptable networks (CANs) containing dynamic β-amino amide moieties have emerged as promising reprocessable polymer networks, combining mechanical robustness with recyclability. In this work, we elucidate the exchange kinetics of both the well-established (retro)-aza-Michael addition and a newly identified transamidation pathway that is operative in β-amino amides. Systematic catalyst screening reveals that acidic catalysts significantly enhance viscoelastic control, thereby improving (re)processing efficiency. Furthermore, we introduce a chemical recycling protocol that enables the recovery of the original amino building blocks with up to 86% purity, demonstrating their direct use as feedstock in material (re)synthesis. These insights advance the fundamental understanding of dynamic bond exchange in β-amino amide based CANs and establish a viable route towards circular thermoset materials for numerous applications.
Abstract The growing environmental challenge of non-recyclable thermosets underscores the urgent need for sustainable alternatives. Covalent adaptable networks (CANs) containing dynamic β-amino amide moieties have emerged as promising reprocessable polymer networks, combining mechanical robustness with recyclability. In this work, we elucidate the exchange kinetics of both the well-established (retro)-aza-Michael addition and a newly identified transamidation pathway that is operative in β-amino amides. Systematic catalyst screening reveals that acidic catalysts significantly enhance viscoelastic control, thereby improving (re)processing efficiency. Furthermore, we introduce a chemical recycling protocol that enables the recovery of the original amino building blocks with up to 86% purity, demonstrating their direct use as feedstock in material (re)synthesis. These insights advance the fundamental understanding of dynamic bond exchange in β-amino amide based CANs and establish a viable route towards circular thermoset materials for numerous applications.
From β-Dicarbonyl Chemistry to Dynamic Polymers
Y. Ma ; C. Weder ; F.E. Du Prez ; J. Augusto Berrocal
Chem. Rev., 2025
Y. Ma ; C. Weder ; F.E. Du Prez ; J. Augusto Berrocal
Chem. Rev., 2025
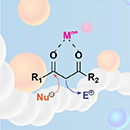 Abstract The past two decades have witnessed an explosion of the use of dynamic bonds in polymer science. The β-dicarbonyl skeleton has emerged as a most versatile platform motif that has been utilized to synthesize a plethora of dynamic polymers that leverage either reversible metal–ligand coordination or exchangeable dynamic covalent bonds. The high modularity and intrinsic dynamic nature of the structures based on the β-dicarbonyl motif have received considerable interest across diverse fields, in applications that include drug delivery, the development of sustainable polymers, 3D printing, actuators, and many others. This review summarizes the progress on dynamic polymers derived from β-dicarbonyl synthons and focuses on three main topics. The first section provides a comprehensive overview of the prevalent methodologies employed for the preparation of polymers containing β-dicarbonyl moieties. The second part highlights the key features, development, and applications of dynamic polymers based on the β-dicarbonyl chemistry, including metallo-supramolecular polymers and dynamic covalent polymer networks. In the concluding section, we offer our views on the future challenges and prospects pertaining to this class of dynamic polymer systems.
Abstract The past two decades have witnessed an explosion of the use of dynamic bonds in polymer science. The β-dicarbonyl skeleton has emerged as a most versatile platform motif that has been utilized to synthesize a plethora of dynamic polymers that leverage either reversible metal–ligand coordination or exchangeable dynamic covalent bonds. The high modularity and intrinsic dynamic nature of the structures based on the β-dicarbonyl motif have received considerable interest across diverse fields, in applications that include drug delivery, the development of sustainable polymers, 3D printing, actuators, and many others. This review summarizes the progress on dynamic polymers derived from β-dicarbonyl synthons and focuses on three main topics. The first section provides a comprehensive overview of the prevalent methodologies employed for the preparation of polymers containing β-dicarbonyl moieties. The second part highlights the key features, development, and applications of dynamic polymers based on the β-dicarbonyl chemistry, including metallo-supramolecular polymers and dynamic covalent polymer networks. In the concluding section, we offer our views on the future challenges and prospects pertaining to this class of dynamic polymer systems.
Debondable polyurethane-inspired adhesives using malonate-formed amide chemistry
T. Maiheu; H.Kassem; A. Roig; N. Kolb; A. Azzawi; F.E. Du Prez
Eur. Polym. J., 2025
T. Maiheu; H.Kassem; A. Roig; N. Kolb; A. Azzawi; F.E. Du Prez
Eur. Polym. J., 2025
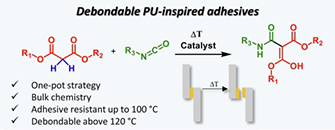 Abstract Polyurethane-based adhesives are widely used to adhere various substrates in a wide range of industrial sectors. While such adhesives are often designed for high performance applications, they present a challenge when it comes to recycling or repairing as a result of their thermoset nature. Here, we explore the potential of malonate-formed amides as thermally triggerable dynamic moieties for the development of debondable adhesives. These malonate-formed amides are introduced in polyurethane-inspired adhesives by the fast and straightforward reaction between available malonate-containing polyesters and diisocyanates. First, a systematic catalyst screening has been performed to evaluate the formation of malonate-formed amides from diethylmalonate and 4-ethylphenylisocyanate. Then, it has been confirmed on material level that the dissociation pathway of the debonding reaction occurs via a decarboxylation pathway without the release of isocyanates. Subsequently, the potential of this reverse reaction is demonstrated for a single debonding, using lap shear testing at debonding temperatures above 120 °C. Overall, this approach enables a chemically designed, industrially attractive generation of debondable adhesives based on malonate-functionalized polyesters.
Abstract Polyurethane-based adhesives are widely used to adhere various substrates in a wide range of industrial sectors. While such adhesives are often designed for high performance applications, they present a challenge when it comes to recycling or repairing as a result of their thermoset nature. Here, we explore the potential of malonate-formed amides as thermally triggerable dynamic moieties for the development of debondable adhesives. These malonate-formed amides are introduced in polyurethane-inspired adhesives by the fast and straightforward reaction between available malonate-containing polyesters and diisocyanates. First, a systematic catalyst screening has been performed to evaluate the formation of malonate-formed amides from diethylmalonate and 4-ethylphenylisocyanate. Then, it has been confirmed on material level that the dissociation pathway of the debonding reaction occurs via a decarboxylation pathway without the release of isocyanates. Subsequently, the potential of this reverse reaction is demonstrated for a single debonding, using lap shear testing at debonding temperatures above 120 °C. Overall, this approach enables a chemically designed, industrially attractive generation of debondable adhesives based on malonate-functionalized polyesters.
Transparent Polyurethane Coating with Selenonium Salt-Enhanced Healing and Antibacterial Properties
S-S. Chen; V. Scholiers; H. Chen; X-W. An; J-J. Li; J. Zhu; F.E. Du Prez; X-Q. Pan
Chin. J. Polym. Sci., 2025
S-S. Chen; V. Scholiers; H. Chen; X-W. An; J-J. Li; J. Zhu; F.E. Du Prez; X-Q. Pan
Chin. J. Polym. Sci., 2025
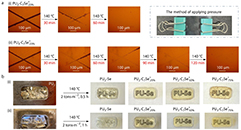 Abstract In this study, dynamic selenonium salts were incorporated into a polyurethane (PU) matrix to develop transparent, healable and antibacterial coatings. Through systematic formulation optimization, optically clear materials with excellent room-temperature hardness were obtained. Fine-tuning the selenonium content established a synergy between antibacterial performance and network dynamics, as evidenced by vitrimer-like rheological behavior at elevated temperatures. Consequently, the coatings exhibited outstanding reprocessability while maintaining high transparency and structural stability after prolonged saltwater exposure. These integrated features underscore the potential of the developed cationic PU coatings as robust, multifunctional materials for electronic device protection and marine antifouling, combining long-term transparency, recyclability, and antibacterial durability.
Abstract In this study, dynamic selenonium salts were incorporated into a polyurethane (PU) matrix to develop transparent, healable and antibacterial coatings. Through systematic formulation optimization, optically clear materials with excellent room-temperature hardness were obtained. Fine-tuning the selenonium content established a synergy between antibacterial performance and network dynamics, as evidenced by vitrimer-like rheological behavior at elevated temperatures. Consequently, the coatings exhibited outstanding reprocessability while maintaining high transparency and structural stability after prolonged saltwater exposure. These integrated features underscore the potential of the developed cationic PU coatings as robust, multifunctional materials for electronic device protection and marine antifouling, combining long-term transparency, recyclability, and antibacterial durability.
Low-Viscosity, Dynamic Amidoamine Hardeners with Tunable Curing Kinetics for Epoxy Adhesives
J. Debuyck ; B. Daelman ; G. Scurani ; S.M. Fischer ; F.E. Du Prez
Macromol., 2025
J. Debuyck ; B. Daelman ; G. Scurani ; S.M. Fischer ; F.E. Du Prez
Macromol., 2025
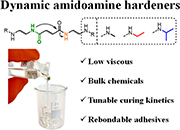 Abstract To address the irreversible cross-linking of amidoamine (AA) hardeners, dynamic alternatives have been developed, but high viscosities and nontunable curing kinetics limit practical applications. We present a novel strategy to reduce the viscosity of dynamic AAs by several orders of magnitude and simultaneously tune their curing kinetics with epoxy compounds. Specifically, polysuccinamide- and polyglutarimide-based curing agents were synthesized from bulk chemicals and end-capped with secondary amine functionalities. The curing kinetics of the amidoamine–epoxy system were tuned via the chemical environment of the secondary amine, while the viscosity of the hardeners was controlled by varying the average amidoamine chain length. The versatility of these dynamic hardeners was investigated in conventional bulk adhesive formulations. Tensile and lap-shear experiments on these resins demonstrated strong adhesion (up to 11 MPa) and excellent rebonding capabilities. This straightforward synthetic strategy enables an attractive drop-in technology toward rebondable amidoamine–epoxy hardeners, starting from low-viscosity formulations with tunable curing kinetics.
Abstract To address the irreversible cross-linking of amidoamine (AA) hardeners, dynamic alternatives have been developed, but high viscosities and nontunable curing kinetics limit practical applications. We present a novel strategy to reduce the viscosity of dynamic AAs by several orders of magnitude and simultaneously tune their curing kinetics with epoxy compounds. Specifically, polysuccinamide- and polyglutarimide-based curing agents were synthesized from bulk chemicals and end-capped with secondary amine functionalities. The curing kinetics of the amidoamine–epoxy system were tuned via the chemical environment of the secondary amine, while the viscosity of the hardeners was controlled by varying the average amidoamine chain length. The versatility of these dynamic hardeners was investigated in conventional bulk adhesive formulations. Tensile and lap-shear experiments on these resins demonstrated strong adhesion (up to 11 MPa) and excellent rebonding capabilities. This straightforward synthetic strategy enables an attractive drop-in technology toward rebondable amidoamine–epoxy hardeners, starting from low-viscosity formulations with tunable curing kinetics.
Comparing Triaminononane and TREN as Trifunctional Amine Cross-Linkers in Covalent Adaptable Networks
J. Debuyck; R. Wink; B. Daelman; R.P. Sijbesma; F.E. Du Prez
ACS Polym. Au, 2025
J. Debuyck; R. Wink; B. Daelman; R.P. Sijbesma; F.E. Du Prez
ACS Polym. Au, 2025
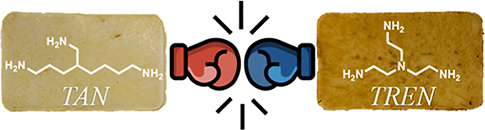 Abstract Primary amines are of utmost importance for the design of various polymeric materials, because of their reactivity, low cost and widespread availability. This is demonstrated by the numerous cross-linking strategies of thermosets that rely on the reaction of multifunctional amines with for example epoxides, esters or aldehydes. Tris(2-aminoethyl)amine (TREN) has long remained the only large-scale available, low mass trifunctional primary amine. Despite its known toxicity, significant vapor pressure and several drawbacks related to its tertiary amine functionality, including oxidation-induced coloration, reduced thermal stability and increased reactivity, TREN helped to shape the field of covalent adaptable networks (CANs). On the other hand, we anticipated that triaminononane (TAN) as an alternative low-viscosity trifunctional primary amine would not face the same difficulties because of its fully aliphatic structure.
Abstract Primary amines are of utmost importance for the design of various polymeric materials, because of their reactivity, low cost and widespread availability. This is demonstrated by the numerous cross-linking strategies of thermosets that rely on the reaction of multifunctional amines with for example epoxides, esters or aldehydes. Tris(2-aminoethyl)amine (TREN) has long remained the only large-scale available, low mass trifunctional primary amine. Despite its known toxicity, significant vapor pressure and several drawbacks related to its tertiary amine functionality, including oxidation-induced coloration, reduced thermal stability and increased reactivity, TREN helped to shape the field of covalent adaptable networks (CANs). On the other hand, we anticipated that triaminononane (TAN) as an alternative low-viscosity trifunctional primary amine would not face the same difficulties because of its fully aliphatic structure.
Combinatorial Synthesis and Evaluation of Trialkyl Galloyl Amidoamine Ionizable Lipids for mRNA Formulation
B. Golba; I. De Franceschi; Z. Zhong; M.J. Schuijs; C.M. Brenis Gomez; H. Lauwers; B. Louage; I.Sheshi; N. Badi; B.G. De Geest; F.E. Du Prez
J. Am. Chem. Soc., 2025
B. Golba; I. De Franceschi; Z. Zhong; M.J. Schuijs; C.M. Brenis Gomez; H. Lauwers; B. Louage; I.Sheshi; N. Badi; B.G. De Geest; F.E. Du Prez
J. Am. Chem. Soc., 2025
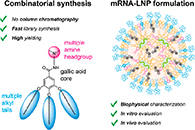 Abstract Lipid nanoparticles (LNPs), containing ionizable cationic lipids, have attracted widespread interest in recent years, particularly following their use as mRNA delivery systems for COVID-19 vaccines. Here, we report on the combinatorial synthesis of galloyl amidoamine-based ionizable lipids. Starting from methyl gallate, three alkyl tails were substituted onto the aromatic ring, and the carboxylic acid was transformed into an ionizable tertiary amine headgroup. Optimization of the synthetic protocol resulted in a scalable, chromatography-free procedure requiring as few as two transformation steps and yielding a library of 43 different lipids in high yield (>88%). By varying the ionizable amine headgroup and the length, saturation, and branching of the alkyl tails, we found that the length of the lipid tail significantly impacted solubility and mRNA encapsulation efficiency. Trialkyl lipids comprising unsaturated heptyl and octyl tails enabled the formulation of sub-150 nm LNPs with encapsulation efficiencies exceeding 85%. Benchmarking experiments against a commercial MC3 LNP formulation identified four lipids that enabled potent mRNA transfection in vitro. Moreover, in vivo studies in mice with selected LNP formulations indicated that three lipids performed on par with MC3 in terms of luciferase reporter-protein expression in the liver and spleen after intravenous administration.
Abstract Lipid nanoparticles (LNPs), containing ionizable cationic lipids, have attracted widespread interest in recent years, particularly following their use as mRNA delivery systems for COVID-19 vaccines. Here, we report on the combinatorial synthesis of galloyl amidoamine-based ionizable lipids. Starting from methyl gallate, three alkyl tails were substituted onto the aromatic ring, and the carboxylic acid was transformed into an ionizable tertiary amine headgroup. Optimization of the synthetic protocol resulted in a scalable, chromatography-free procedure requiring as few as two transformation steps and yielding a library of 43 different lipids in high yield (>88%). By varying the ionizable amine headgroup and the length, saturation, and branching of the alkyl tails, we found that the length of the lipid tail significantly impacted solubility and mRNA encapsulation efficiency. Trialkyl lipids comprising unsaturated heptyl and octyl tails enabled the formulation of sub-150 nm LNPs with encapsulation efficiencies exceeding 85%. Benchmarking experiments against a commercial MC3 LNP formulation identified four lipids that enabled potent mRNA transfection in vitro. Moreover, in vivo studies in mice with selected LNP formulations indicated that three lipids performed on par with MC3 in terms of luciferase reporter-protein expression in the liver and spleen after intravenous administration.
Precision Photochemistry: Every Photon Counts
F. Pashley-Johnson; X. Wu; J.A. Carroll; S.L. Walden; H. Frisch; A.-N. Unterreiner; F.E. Du Prez; H.-A. Wagenknecht; J. Read de Alaniz; B.L. Feringa; A. Heckel; C. Barner-Kowollik
Angew. Chem. Int. Ed., 2025
F. Pashley-Johnson; X. Wu; J.A. Carroll; S.L. Walden; H. Frisch; A.-N. Unterreiner; F.E. Du Prez; H.-A. Wagenknecht; J. Read de Alaniz; B.L. Feringa; A. Heckel; C. Barner-Kowollik
Angew. Chem. Int. Ed., 2025
 Abstract Photochemistry is undergoing a precision transformation. Through technological advancements, such as the advent of light emitting diodes and monochromatic lasers, chemists are now able to use photons not only as an energy source but also as a tool for directing photochemical processes with both wavelength and spatiotemporal precision. Enabled by these technologies, the discovery that photochemical action often does not align with molar extinction has catalysed the growth of the research field that we coin Precision Photochemistry. We explain how precision photochemistry stands on four fundamental pillars: molar extinction, wavelength-dependent quantum yield, concentration of the chromophores, and the length of the irradiation. Each of these four pillars are intrinsically linked and dictate the experimental conditions that should be used (e.g., wavelength, light intensity, and solvent system), as we demonstrate through simulations of a photochemical uncaging system. Building on these pillars, we propose a concrete definition for Precision Photochemistry and highlight important fields within chemistry that will benefit from careful consideration of them. Finally, we address key experimental considerations that must be taken into account when conducting precision photochemistry including the light source, the reaction setup, and the method for determining (wavelength-dependent) quantum yields. These factors are critical in furthering the development of the field of Precision Photochemistry.
Abstract Photochemistry is undergoing a precision transformation. Through technological advancements, such as the advent of light emitting diodes and monochromatic lasers, chemists are now able to use photons not only as an energy source but also as a tool for directing photochemical processes with both wavelength and spatiotemporal precision. Enabled by these technologies, the discovery that photochemical action often does not align with molar extinction has catalysed the growth of the research field that we coin Precision Photochemistry. We explain how precision photochemistry stands on four fundamental pillars: molar extinction, wavelength-dependent quantum yield, concentration of the chromophores, and the length of the irradiation. Each of these four pillars are intrinsically linked and dictate the experimental conditions that should be used (e.g., wavelength, light intensity, and solvent system), as we demonstrate through simulations of a photochemical uncaging system. Building on these pillars, we propose a concrete definition for Precision Photochemistry and highlight important fields within chemistry that will benefit from careful consideration of them. Finally, we address key experimental considerations that must be taken into account when conducting precision photochemistry including the light source, the reaction setup, and the method for determining (wavelength-dependent) quantum yields. These factors are critical in furthering the development of the field of Precision Photochemistry.
Scalable β-Aminoester-Based Covalent Adaptable Networks for Wind Turbine Blade Composites
S.M. Fischer; I. De Baere; L.Y. Nguyen; H. Stecher; W. Van Paepegem; F.E. Du Prez
Macromol., 2025
S.M. Fischer; I. De Baere; L.Y. Nguyen; H. Stecher; W. Van Paepegem; F.E. Du Prez
Macromol., 2025
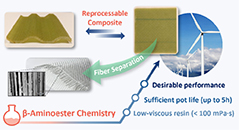 Abstract Wind turbine blades pose a major recycling challenge due to their complex composition of thermoset polymers embedded in fiber-reinforced composites. This study presents a cost-effective and scalable approach using covalent adaptable networks based on β-aminoester curing agents for epoxy resins, specifically tailored to meet the requirements of the wind turbine blade industry. The formulation offers low mixed viscosity (<100 mPa·s) and customizable pot life of up to more than 5 h and provides glass transition temperatures of above 75 °C and tensile stiffness exceeding 2.8 GPa. Furthermore, the successful reshaping of glass fiber-reinforced composites, produced by vacuum-assisted resin infusion, and the feasibility of chemically degrading the polymer matrix with acetic acid have been demonstrated. The herein-presented approach holds promise for advancing sustainable practices in wind energy infrastructure.
Abstract Wind turbine blades pose a major recycling challenge due to their complex composition of thermoset polymers embedded in fiber-reinforced composites. This study presents a cost-effective and scalable approach using covalent adaptable networks based on β-aminoester curing agents for epoxy resins, specifically tailored to meet the requirements of the wind turbine blade industry. The formulation offers low mixed viscosity (<100 mPa·s) and customizable pot life of up to more than 5 h and provides glass transition temperatures of above 75 °C and tensile stiffness exceeding 2.8 GPa. Furthermore, the successful reshaping of glass fiber-reinforced composites, produced by vacuum-assisted resin infusion, and the feasibility of chemically degrading the polymer matrix with acetic acid have been demonstrated. The herein-presented approach holds promise for advancing sustainable practices in wind energy infrastructure.
Poly(styrene-co-maleamic acid)-based monoamide covalent adaptable networks
Aitor Hernández; S.M. Fischer; J.M. Winne; F.E. Du Prez
J. Mater. Chem. A, 2025
Aitor Hernández; S.M. Fischer; J.M. Winne; F.E. Du Prez
J. Mater. Chem. A, 2025
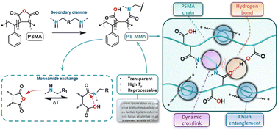 Abstract This study presents poly(styrene-co-maleamic acid)-based monoamide (PS-MMA) covalent adaptable networks (CANs) as a novel class of high performance dynamic covalent polymer networks. PS-MMA CANs are readily synthesized by crosslinking poly(styrene-co-maleic anhydride) (PSMA) copolymers with secondary diamines, introducing a previously unexplored dynamic monoamide exchange chemistry. By tailoring the amine-to-anhydride ratios, crosslink density and viscoelastic properties were finely adjusted, yielding networks with high thermal stability and reprocessability. The dissociation of monoamides into amines and anhydrides, as observed in high-temperature FT-IR analysis, was validated through Density Functional Theory (DFT) calculations. These calculations revealed an enthalpically favored tendency for amines and anhydrides to re-associate into monoamides, confirming their suitability for thermally triggered dynamics and effective viscosity control at increasing temperatures. Rheological analysis of the PS-MMA CANs showed distinct diamine structure-dependent profiles, where the interplay between the chain entanglements, supramolecular interactions and dynamic dissociative monoamide debonding governed their stress relaxation regimes and macroscopic flow behavior.
Abstract This study presents poly(styrene-co-maleamic acid)-based monoamide (PS-MMA) covalent adaptable networks (CANs) as a novel class of high performance dynamic covalent polymer networks. PS-MMA CANs are readily synthesized by crosslinking poly(styrene-co-maleic anhydride) (PSMA) copolymers with secondary diamines, introducing a previously unexplored dynamic monoamide exchange chemistry. By tailoring the amine-to-anhydride ratios, crosslink density and viscoelastic properties were finely adjusted, yielding networks with high thermal stability and reprocessability. The dissociation of monoamides into amines and anhydrides, as observed in high-temperature FT-IR analysis, was validated through Density Functional Theory (DFT) calculations. These calculations revealed an enthalpically favored tendency for amines and anhydrides to re-associate into monoamides, confirming their suitability for thermally triggered dynamics and effective viscosity control at increasing temperatures. Rheological analysis of the PS-MMA CANs showed distinct diamine structure-dependent profiles, where the interplay between the chain entanglements, supramolecular interactions and dynamic dissociative monoamide debonding governed their stress relaxation regimes and macroscopic flow behavior.
Eliminating creep in vitrimers using temperature-resilient siloxane exchange chemistry and N-heterocyclic carbenes
T. Debsharma; L.T. Nguyen; B.P. Maliszewski; S.M. Fischer; V. Scholiers; J.M. Winne; S.P. Nolan; F.E. Du Prez
Chem. Sci., 2025
T. Debsharma; L.T. Nguyen; B.P. Maliszewski; S.M. Fischer; V. Scholiers; J.M. Winne; S.P. Nolan; F.E. Du Prez
Chem. Sci., 2025
 Abstract This study explores a novel N-heterocyclic carbene-mediated siloxane exchange mechanism, laying the foundation for designing covalent adaptable networks (CANs) with high temperature stability (>200 °C) for dynamic covalent chemistry. Small molecule siloxane compounds, obtained by hydrosilylation reactions, are used to demonstrate siloxane-exchange via a mechanism supported by density functional theory. The proposed mechanism presents an equilibrium, at elevated temperatures, between an imidazolium salt and its free carbene form, which is the catalytically active species. Following this mechanistic insight, a tetra-substituted ester-terminated siloxane cross-linker was synthesized and cured with a commercial amine hardener. The ensuing ester–amine reaction yields thermally stable, non-dynamic amide bonds, thereby enhancing material stability. The resulting CANs exhibit rapid stress relaxation at elevated temperatures and demonstrate successful recycling through compression molding without any significant loss of material properties. Remarkably, the synthesized material showcases high creep resistance, even up to 150 °C, indicating the benefits of having a thermally reversible catalyst system for siloxane activation.
Abstract This study explores a novel N-heterocyclic carbene-mediated siloxane exchange mechanism, laying the foundation for designing covalent adaptable networks (CANs) with high temperature stability (>200 °C) for dynamic covalent chemistry. Small molecule siloxane compounds, obtained by hydrosilylation reactions, are used to demonstrate siloxane-exchange via a mechanism supported by density functional theory. The proposed mechanism presents an equilibrium, at elevated temperatures, between an imidazolium salt and its free carbene form, which is the catalytically active species. Following this mechanistic insight, a tetra-substituted ester-terminated siloxane cross-linker was synthesized and cured with a commercial amine hardener. The ensuing ester–amine reaction yields thermally stable, non-dynamic amide bonds, thereby enhancing material stability. The resulting CANs exhibit rapid stress relaxation at elevated temperatures and demonstrate successful recycling through compression molding without any significant loss of material properties. Remarkably, the synthesized material showcases high creep resistance, even up to 150 °C, indicating the benefits of having a thermally reversible catalyst system for siloxane activation.
Debondable phenoxy-based structural adhesives with β-amino amide containing reversible crosslinkers
F. Portone; L.T. Nguyen; R. Pinalli; A. Pedrini; F.E. Du Prez; E. Dalcanale
RSC Appl. Polym., 2025
F. Portone; L.T. Nguyen; R. Pinalli; A. Pedrini; F.E. Du Prez; E. Dalcanale
RSC Appl. Polym., 2025
 Abstract This study introduces dynamic phenoxy-based adhesives using β-aminoamide exchange chemistry, designed for durability, reprocessability and sustainability. Synthesized through a two-step process, the adhesive features linear poly-aminoamides and tailored amine formulations to optimize adhesion, flexibility, and the glass transition. The corresponding phenoxy-based adhesives demonstrated effective crosslinking, high thermal stability (Td5% ∼340–350 °C), and temperature-responsive viscoelastic properties. Notably, the materials with 5 mol% of TETA (E-BAAT5) exhibited ideal activation energy for stress relaxation, exceptional creep resistance, and retained up to 98% lap shear strength after recycling, with controlled debonding at elevated temperatures, making it ideal for high-performance, recyclable adhesive applications.
Abstract This study introduces dynamic phenoxy-based adhesives using β-aminoamide exchange chemistry, designed for durability, reprocessability and sustainability. Synthesized through a two-step process, the adhesive features linear poly-aminoamides and tailored amine formulations to optimize adhesion, flexibility, and the glass transition. The corresponding phenoxy-based adhesives demonstrated effective crosslinking, high thermal stability (Td5% ∼340–350 °C), and temperature-responsive viscoelastic properties. Notably, the materials with 5 mol% of TETA (E-BAAT5) exhibited ideal activation energy for stress relaxation, exceptional creep resistance, and retained up to 98% lap shear strength after recycling, with controlled debonding at elevated temperatures, making it ideal for high-performance, recyclable adhesive applications.
Synthesis of triamine-functionalized rigid crosslinkers for materials science
N. Braidi; A. Hernández; G. Scurani; F. Parenti; N. Badi; F.E. Du Prez
Polym. Chem., 2025
N. Braidi; A. Hernández; G. Scurani; F. Parenti; N. Badi; F.E. Du Prez
Polym. Chem., 2025
 Abstract In this study, a primary amine-terminated star-shaped polystyrene (PS) was synthesized using an Activators Regenerated by Electron Transfer Atom Transfer Radical Polymerization (ARGET ATRP) protocol, yielding products with low dispersity (<1.2) and molar masses in the range of 2 to 12 kDa. The influence of the trifunctional initiator's reactivity on the resulting polymer topology was investigated. The bromo-terminated PS was efficiently converted to its azide-terminated counterpart as confirmed by online ATR FT-IR and NMR spectroscopy. The targeted amine-terminated PS was then obtained by a Staudinger reduction of the azide groups using tributylphosphine. To assess the applicability of these novel amine-terminated PSs as well-defined trifunctional crosslinking agents, traditional epoxy thermoset networks and covalent adaptable networks (CANs) were synthesized using diepoxides or diacetoacetates, respectively. The resulting materials exhibited excellent thermal resistance, attributed to the high PS content. Moreover, by making use of the option of tuning the molar mass of such macromolecular crosslinkers, the network's crosslinking density could be tailored, enabling control over swelling degree, glass transition temperature, and, in the case of the obtained vinylogous urethane vitrimers, even reprocessability.
Abstract In this study, a primary amine-terminated star-shaped polystyrene (PS) was synthesized using an Activators Regenerated by Electron Transfer Atom Transfer Radical Polymerization (ARGET ATRP) protocol, yielding products with low dispersity (<1.2) and molar masses in the range of 2 to 12 kDa. The influence of the trifunctional initiator's reactivity on the resulting polymer topology was investigated. The bromo-terminated PS was efficiently converted to its azide-terminated counterpart as confirmed by online ATR FT-IR and NMR spectroscopy. The targeted amine-terminated PS was then obtained by a Staudinger reduction of the azide groups using tributylphosphine. To assess the applicability of these novel amine-terminated PSs as well-defined trifunctional crosslinking agents, traditional epoxy thermoset networks and covalent adaptable networks (CANs) were synthesized using diepoxides or diacetoacetates, respectively. The resulting materials exhibited excellent thermal resistance, attributed to the high PS content. Moreover, by making use of the option of tuning the molar mass of such macromolecular crosslinkers, the network's crosslinking density could be tailored, enabling control over swelling degree, glass transition temperature, and, in the case of the obtained vinylogous urethane vitrimers, even reprocessability.
Taking dynamic covalent chemistry out of the lab and into reprocessable industrial thermosets
S. Maes; N. Badi; J.M. Winne; F.E. Du Prez
Nat. Rev. Chem., 2025
S. Maes; N. Badi; J.M. Winne; F.E. Du Prez
Nat. Rev. Chem., 2025
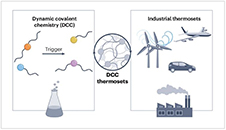 Abstract Dynamic covalent chemistry (DCC) allows the development of thermally (re)processable and recyclable polymer networks, which is a highly attractive feature for new generations of thermoset materials. However, despite a surge in academic interest wherein soon almost any imaginable DCC platform may have been applied in a thermoset formulation, dynamic or reversible covalent polymer networks have so far found only few industrial applications. This Review provides a perspective on the main strategies for the application of DCC in the design and development of bulk thermoset materials, and it presents some of the key hurdles for their industrial implementation. The polymer design strategies and associated chemistries are viewed from the perspective of how ‘close to market’ their development pathway is, thus providing a roadmap to achieve high-volume breakthrough applications.
Abstract Dynamic covalent chemistry (DCC) allows the development of thermally (re)processable and recyclable polymer networks, which is a highly attractive feature for new generations of thermoset materials. However, despite a surge in academic interest wherein soon almost any imaginable DCC platform may have been applied in a thermoset formulation, dynamic or reversible covalent polymer networks have so far found only few industrial applications. This Review provides a perspective on the main strategies for the application of DCC in the design and development of bulk thermoset materials, and it presents some of the key hurdles for their industrial implementation. The polymer design strategies and associated chemistries are viewed from the perspective of how ‘close to market’ their development pathway is, thus providing a roadmap to achieve high-volume breakthrough applications.
Interlaminar fracture toughness behaviour of a repairable glass-fibre-reinforced vitrimer for wind-energy applications
V. Amfilochiou, T. Debsharma, I. De Baere, F.E. Du Prez, W. Van Paepegem
Compos. B: Eng, 2025
V. Amfilochiou, T. Debsharma, I. De Baere, F.E. Du Prez, W. Van Paepegem
Compos. B: Eng, 2025
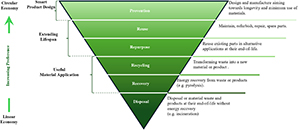 Abstract During the life of a composite part, the need of repair due to delamination may arise, especially since the reuse of composites is being promoted as a sustainable management solution for composite waste. For thermoset composite parts, which are commonly used for wind-energy blades’ manufacturing, the repair process can be costly and long as the damaged area needs to be removed and replaced. In the present work, a siloxane-based-vitrimer composite that provides the advantage of healing by hot-pressing for only 40 min, was investigated as an alternative to conventional infusible thermoset composites. The quality of the optimised repair cycle was evaluated by optical microscopy and X-ray tomography. The mechanical performance of the vitrimer composite was investigated under Mode I and Mode II fracture by measuring the respective toughness prior and after repair in comparison to an industrial thermoset benchmark. The results demonstrated that the vitrimer composite exhibits fracture toughness values that are comparable to the benchmark, while reaching >89 % repair efficiency restoring its GIC and GIIC and making it a viable alternative to thermoset structural composites. The above results represent a breakthrough in the design of future sustainable composites based on epoxy and infusible systems for wind-energy applications.
Abstract During the life of a composite part, the need of repair due to delamination may arise, especially since the reuse of composites is being promoted as a sustainable management solution for composite waste. For thermoset composite parts, which are commonly used for wind-energy blades’ manufacturing, the repair process can be costly and long as the damaged area needs to be removed and replaced. In the present work, a siloxane-based-vitrimer composite that provides the advantage of healing by hot-pressing for only 40 min, was investigated as an alternative to conventional infusible thermoset composites. The quality of the optimised repair cycle was evaluated by optical microscopy and X-ray tomography. The mechanical performance of the vitrimer composite was investigated under Mode I and Mode II fracture by measuring the respective toughness prior and after repair in comparison to an industrial thermoset benchmark. The results demonstrated that the vitrimer composite exhibits fracture toughness values that are comparable to the benchmark, while reaching >89 % repair efficiency restoring its GIC and GIIC and making it a viable alternative to thermoset structural composites. The above results represent a breakthrough in the design of future sustainable composites based on epoxy and infusible systems for wind-energy applications.
Tailoring the Reprocessability of Thiol-Ene Networks through Ring Size Effects
V. Scholiers; S.M. Fischer; B. Daelman; S. Lehner; S.Gaan; J.M. Winne; F.E. Du Prez
Angew. Chem., 2025
V. Scholiers; S.M. Fischer; B. Daelman; S. Lehner; S.Gaan; J.M. Winne; F.E. Du Prez
Angew. Chem., 2025
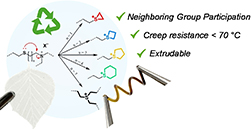 Abstract Recycling thermosetting materials presents itself as a major challenge in achieving sustainable material use. Dynamic covalent cross-linking of polymers has emerged as a viable solution that can combine the structural integrity of thermosetting materials with the (re−)processability of thermoplastics. Thioether linkages between polymer chains are quite common, and their use dates back to the vulcanization of rubbers. While it is known that thioether bonds can be triggered to exchange through transalkylation reactions, this process is usually slow, as thioether moieties not only have to be activated by an alkylating agent, but the activated thioether also has to associate with a second thioether moiety in a classical SN2-type process. Here, we present the rational design of dynamic polymer networks based on simple dithiol-based monomers and a fatty acid derived triene. Two neighboring thioethers can undergo a much faster bond exchange reaction, and we found that the exchange dynamics can be further tuned over almost three orders of magnitude by tailoring the distance between two thioether functionalities. This resulted in thioether-cross-linked materials that could be processed by extrusion, a continuous reprocessing technique that was previously not accessible for this class of cross-linked materials, while still exhibiting appealing creep-resistance below 70 °C.
Abstract Recycling thermosetting materials presents itself as a major challenge in achieving sustainable material use. Dynamic covalent cross-linking of polymers has emerged as a viable solution that can combine the structural integrity of thermosetting materials with the (re−)processability of thermoplastics. Thioether linkages between polymer chains are quite common, and their use dates back to the vulcanization of rubbers. While it is known that thioether bonds can be triggered to exchange through transalkylation reactions, this process is usually slow, as thioether moieties not only have to be activated by an alkylating agent, but the activated thioether also has to associate with a second thioether moiety in a classical SN2-type process. Here, we present the rational design of dynamic polymer networks based on simple dithiol-based monomers and a fatty acid derived triene. Two neighboring thioethers can undergo a much faster bond exchange reaction, and we found that the exchange dynamics can be further tuned over almost three orders of magnitude by tailoring the distance between two thioether functionalities. This resulted in thioether-cross-linked materials that could be processed by extrusion, a continuous reprocessing technique that was previously not accessible for this class of cross-linked materials, while still exhibiting appealing creep-resistance below 70 °C.
Enabling the Reprocessability and Debonding of Epoxy Thermosets Using Dynamic Poly(β-Amino Amide) Curing Agents
L.T. Nguyen; S. Maes; F.E. Du Prez
Adv. Funct. Mater., 2025
L.T. Nguyen; S. Maes; F.E. Du Prez
Adv. Funct. Mater., 2025
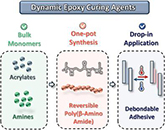 Abstract Epoxy resins, by showing outstanding performances, stand out as the most applied materials in thermoset products. However, their excellent properties, associated with covalently cross-linked structures, come at the expense of recyclability, thus posing environmental and regulatory challenges. Herein, starting from the recently explored reversibility of robust poly(β-amino amide)s, dynamic curing agents are synthesized in a one-pot procedure for their use in the preparation of epoxy-derived dynamic networks. The obtained materials retain desirable properties while being fully (re)processable, with high temperature-dependent viscoelasticity (activation energy (Ea) of ≈230 to 270 kJ mol−1). Moreover, this new generation of epoxy materials shows excellent resistance to hydrolysis and creep at elevated temperatures (up to 120 °C). As an entry point to further applications, the reversible curing agents are implemented in adhesive formulations, showcasing lap shear strengths that are comparable to commercial hardeners (up to 9 MPa). The β-amino amide groups provide the obtained adhesives with the additional functionality of heat-triggered deconstruction at elevated temperatures (130 to 150 °C), and re-bonding capacity with up to 80% recovery in lap-shear strength. To encourage industrial adoption, a cost-effective, drop-in synthesis protocol is developed using only bulk chemicals, hence facilitating practical implementation.
Abstract Epoxy resins, by showing outstanding performances, stand out as the most applied materials in thermoset products. However, their excellent properties, associated with covalently cross-linked structures, come at the expense of recyclability, thus posing environmental and regulatory challenges. Herein, starting from the recently explored reversibility of robust poly(β-amino amide)s, dynamic curing agents are synthesized in a one-pot procedure for their use in the preparation of epoxy-derived dynamic networks. The obtained materials retain desirable properties while being fully (re)processable, with high temperature-dependent viscoelasticity (activation energy (Ea) of ≈230 to 270 kJ mol−1). Moreover, this new generation of epoxy materials shows excellent resistance to hydrolysis and creep at elevated temperatures (up to 120 °C). As an entry point to further applications, the reversible curing agents are implemented in adhesive formulations, showcasing lap shear strengths that are comparable to commercial hardeners (up to 9 MPa). The β-amino amide groups provide the obtained adhesives with the additional functionality of heat-triggered deconstruction at elevated temperatures (130 to 150 °C), and re-bonding capacity with up to 80% recovery in lap-shear strength. To encourage industrial adoption, a cost-effective, drop-in synthesis protocol is developed using only bulk chemicals, hence facilitating practical implementation.
Activated Phenyl Ester Vitrimers
S. Engelen; B. Daelman; J.M. Winne; F.E. Du Prez
Macromol. Rapid Commun., 2025
S. Engelen; B. Daelman; J.M. Winne; F.E. Du Prez
Macromol. Rapid Commun., 2025
.png) Abstract Aromatic esters are amongst the oldest known chemical motifs that allow for thermal (re)processing of thermosetting polymers. Moreover, phenyl esters are generally known as activated esters that do not require a catalyst to undergo acyl transfer reactions. Even though dynamic aromatic esters find applications in commercialized thermoset formulations, all-aromatic esters have found limited use so far in the design of covalent adaptable networks (CAN) as a result of their high glass transition temperature (Tg) and specific curing process. Here, a strategy to include partly aromatic esters as dynamic cross-links inside low Tg (−40 °C) thermosetting formulations, using aliphatic esters derived from para-hydroxybenzoic acid, which serves as a highly activated phenol or as a reactive “phenylogous anhydride” is reported. A small molecule study shows that the activated phenyl ester bonds can readily exchange with free phenol moieties at 200 °C under catalyst-free conditions, while the addition of a catalyst allows for a faster exchange. Robust and hydrophobic polymer networks are conveniently prepared via rapid thiol-ene UV-curing of unsaturated phenol esters. The obtained networks show high thermal stability (350 °C), fast processability, good water resistance, and low creep up to 120 °C, thus showing good promise as a platform for CAN.
Abstract Aromatic esters are amongst the oldest known chemical motifs that allow for thermal (re)processing of thermosetting polymers. Moreover, phenyl esters are generally known as activated esters that do not require a catalyst to undergo acyl transfer reactions. Even though dynamic aromatic esters find applications in commercialized thermoset formulations, all-aromatic esters have found limited use so far in the design of covalent adaptable networks (CAN) as a result of their high glass transition temperature (Tg) and specific curing process. Here, a strategy to include partly aromatic esters as dynamic cross-links inside low Tg (−40 °C) thermosetting formulations, using aliphatic esters derived from para-hydroxybenzoic acid, which serves as a highly activated phenol or as a reactive “phenylogous anhydride” is reported. A small molecule study shows that the activated phenyl ester bonds can readily exchange with free phenol moieties at 200 °C under catalyst-free conditions, while the addition of a catalyst allows for a faster exchange. Robust and hydrophobic polymer networks are conveniently prepared via rapid thiol-ene UV-curing of unsaturated phenol esters. The obtained networks show high thermal stability (350 °C), fast processability, good water resistance, and low creep up to 120 °C, thus showing good promise as a platform for CAN.
Debondable Epoxy-Acrylate Adhesives using β-Amino Ester Chemistry
T. Maiheu; E. Laguzzi; A.T. Slark; F.E. Du Prez
ACS Appl. Mater. Interfaces, 2024
T. Maiheu; E. Laguzzi; A.T. Slark; F.E. Du Prez
ACS Appl. Mater. Interfaces, 2024
.jpg) Abstract The reuse of multilayered materials, which are held together by structural epoxy adhesives, is a major challenge since the bonded substrates cannot be easily separated for recycling. In this research, we explore a one-pot strategy based on β-amino ester chemistry for the development of modified epoxy adhesives with on-demand debonding potential. For this, a formulation of commercially available acrylate, epoxy and amine compounds is used. The research starts with a systematic study, demonstrating the influence of the different compounds on the thermal and adhesive properties of the materials. Subsequently, the potential for debonding is demonstrated using rheological measurements and tensile tests. The fast, catalyst-free Aza-Michael reaction enables the straightforward preparation of such epoxy-based adhesives, while the reverse reaction allows for debonding at 120 °C. In general, a chemical design is demonstrated for producing an industrially attractive generation of debondable epoxy-based adhesives.
Abstract The reuse of multilayered materials, which are held together by structural epoxy adhesives, is a major challenge since the bonded substrates cannot be easily separated for recycling. In this research, we explore a one-pot strategy based on β-amino ester chemistry for the development of modified epoxy adhesives with on-demand debonding potential. For this, a formulation of commercially available acrylate, epoxy and amine compounds is used. The research starts with a systematic study, demonstrating the influence of the different compounds on the thermal and adhesive properties of the materials. Subsequently, the potential for debonding is demonstrated using rheological measurements and tensile tests. The fast, catalyst-free Aza-Michael reaction enables the straightforward preparation of such epoxy-based adhesives, while the reverse reaction allows for debonding at 120 °C. In general, a chemical design is demonstrated for producing an industrially attractive generation of debondable epoxy-based adhesives.
Scalable design of uniform oligourethanes for impact study of chain length, sequence and end groups on thermal properties
J. Van Hoorde; N. Badi; F.E. Du Prez
Polym. Chem., 2024
J. Van Hoorde; N. Badi; F.E. Du Prez
Polym. Chem., 2024
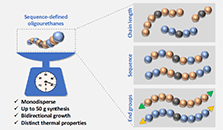 Abstract The full potential of sequence-defined macromolecules remains unexplored, hindered by the difficulty of synthesizing sufficient amounts for the investigation of the properties of such uniform structures and their derived materials. Herein, we report the bidirectional synthesis and thermal behavior analysis of sequence-defined oligourethanes. The synthesis was conducted on a large scale (up to 50 grams) using a straightforward protocol, yielding uniform macromolecules as validated by NMR, ESI-MS and SEC. With this approach, a library of uniform oligourethanes (up to the octamers) was produced using two structural units: a hydrogen-bonding carbamate and a methyl-substituted alternative structure. By varying the chain length, monomer sequence and functionality, we were able to perform a systematic study of the impact of hydrogen bonding on the thermal properties of polyurethanes. Thermal analysis of the discrete oligomers using DSC revealed that both the molecular weight and microstructure significantly affect the glass transition and melting temperatures. TGA measurements also revealed differences in the thermal stability of the oligomers, underscoring the significance of the primary structure of polyurethanes. Additionally, the influence of the terminal groups on the degradation pathway was assessed via pyrolysis-GC-MS, which specifically highlighted the increased thermal stability in the absence of hydroxyl end groups.
Abstract The full potential of sequence-defined macromolecules remains unexplored, hindered by the difficulty of synthesizing sufficient amounts for the investigation of the properties of such uniform structures and their derived materials. Herein, we report the bidirectional synthesis and thermal behavior analysis of sequence-defined oligourethanes. The synthesis was conducted on a large scale (up to 50 grams) using a straightforward protocol, yielding uniform macromolecules as validated by NMR, ESI-MS and SEC. With this approach, a library of uniform oligourethanes (up to the octamers) was produced using two structural units: a hydrogen-bonding carbamate and a methyl-substituted alternative structure. By varying the chain length, monomer sequence and functionality, we were able to perform a systematic study of the impact of hydrogen bonding on the thermal properties of polyurethanes. Thermal analysis of the discrete oligomers using DSC revealed that both the molecular weight and microstructure significantly affect the glass transition and melting temperatures. TGA measurements also revealed differences in the thermal stability of the oligomers, underscoring the significance of the primary structure of polyurethanes. Additionally, the influence of the terminal groups on the degradation pathway was assessed via pyrolysis-GC-MS, which specifically highlighted the increased thermal stability in the absence of hydroxyl end groups.
Direct restoration of photocurable cross-linkers for repeated light-based 3D printing of covalent adaptable networks
L.T. Nguyen; F.E. Du Prez
Mater. Horiz., 2024
L.T. Nguyen; F.E. Du Prez
Mater. Horiz., 2024
.jpg) Abstract Light-based processing of thermosets has gained increasing attention because of its broad application field including its use in digital light processing (DLP) 3D printing. This technique offers efficient design and fabrication of complex structures but typically results in non-recyclable thermoset-based products. To address this issue, we describe here a photocurable, dynamic β-amino ester (BAE) based cross-linker that is not only suitable for DLP printing but can also be chemically degraded via transesterification upon the addition of 2-hydroxyethyl methacrylate (HEMA) as a decross-linker. This conceptually new protocol allows for efficient depolymerization with the direct restoration of curable monomers in a single step without the addition of external catalysts or solvents. By implementing this protocol, we have established a chemical recycling loop for multiple cycles of photo-cross-linking and restoration of cross-linkers, facilitating repeatable DLP 3D printing without generating any waste. The recycled materials exhibit full recovery of thermal properties and Young's modulus while maintaining 75% of their tensile strength for at least three cycles. Simultaneously, the presence of BAE moieties enables the (re)processability of these materials through compression molding.
Abstract Light-based processing of thermosets has gained increasing attention because of its broad application field including its use in digital light processing (DLP) 3D printing. This technique offers efficient design and fabrication of complex structures but typically results in non-recyclable thermoset-based products. To address this issue, we describe here a photocurable, dynamic β-amino ester (BAE) based cross-linker that is not only suitable for DLP printing but can also be chemically degraded via transesterification upon the addition of 2-hydroxyethyl methacrylate (HEMA) as a decross-linker. This conceptually new protocol allows for efficient depolymerization with the direct restoration of curable monomers in a single step without the addition of external catalysts or solvents. By implementing this protocol, we have established a chemical recycling loop for multiple cycles of photo-cross-linking and restoration of cross-linkers, facilitating repeatable DLP 3D printing without generating any waste. The recycled materials exhibit full recovery of thermal properties and Young's modulus while maintaining 75% of their tensile strength for at least three cycles. Simultaneously, the presence of BAE moieties enables the (re)processability of these materials through compression molding.
Foam‐to‐Foam Recycling Potential of PU‐Foams by Integration of Amino Esters
H. Kassem; E.H. Samat; L. Imbernon; F.E. Du Prez
Adv. Funct. Mater., 2024
H. Kassem; E.H. Samat; L. Imbernon; F.E. Du Prez
Adv. Funct. Mater., 2024
 Abstract The increasing market demand for flexible polyurethane foams (PUFs) is prompting the academic and industrial community to seek solutions for their end-of-life management. In this study, this issue is addressed by incorporating β-amino ester (BAE) moieties into the foam structure. This addition results in flexible hybrid PU-BAE foams that can be thermally reprocessed and chemically recycled, with cell sizes ranging from 780 to 990 µm and densities between 90 and 150 kg m−3. These foams demonstrate thermomechanical reprocessability, yielding stable PU-BAE elastomers, with glass transition temperature values ranging from 14 to 25 °C. Stress relaxation experiments reveal remarkably fast relaxation times for these hybrid elastomers (23 s at 160 °C). Furthermore, a systematic investigation of the chemical recyclability of these foams via glycosylation is conducted under mild conditions, achieving complete disintegration within 20 min at 65 °C. Finally, the chemically recycled product is converted into a foam again, achieving a closed loop foam-to-foam recycling process. These findings indicate that the integration of BAE into PUFs leads to an enhanced chemical recycling rate, providing a promising avenue for addressing end-of-life concerns in PUF-applications.
Abstract The increasing market demand for flexible polyurethane foams (PUFs) is prompting the academic and industrial community to seek solutions for their end-of-life management. In this study, this issue is addressed by incorporating β-amino ester (BAE) moieties into the foam structure. This addition results in flexible hybrid PU-BAE foams that can be thermally reprocessed and chemically recycled, with cell sizes ranging from 780 to 990 µm and densities between 90 and 150 kg m−3. These foams demonstrate thermomechanical reprocessability, yielding stable PU-BAE elastomers, with glass transition temperature values ranging from 14 to 25 °C. Stress relaxation experiments reveal remarkably fast relaxation times for these hybrid elastomers (23 s at 160 °C). Furthermore, a systematic investigation of the chemical recyclability of these foams via glycosylation is conducted under mild conditions, achieving complete disintegration within 20 min at 65 °C. Finally, the chemically recycled product is converted into a foam again, achieving a closed loop foam-to-foam recycling process. These findings indicate that the integration of BAE into PUFs leads to an enhanced chemical recycling rate, providing a promising avenue for addressing end-of-life concerns in PUF-applications.
How molecular architecture defines quantum yields
F. Pashley-Johnson; R. Munaweera; S.I. Hossain; S.C. Gauci; L. Delafresnaye; H. Frisch; M.L. O’Mara; F.E. Du Prez; C. Barner-Kowollik
Nat. Commun., 2024
F. Pashley-Johnson; R. Munaweera; S.I. Hossain; S.C. Gauci; L. Delafresnaye; H. Frisch; M.L. O’Mara; F.E. Du Prez; C. Barner-Kowollik
Nat. Commun., 2024
 Abstract Understanding the intricate relationship between molecular architecture and function underpins most challenges at the forefront of chemical innovation. Bond-forming reactions are particularly influenced by the topology of a chemical structure, both on small molecule scale and in larger macromolecular frameworks. Herein, we elucidate the impact that molecular architecture has on the photo-induced cyclisations of a series of monodisperse macromolecules with defined spacers between photodimerisable moieties, and examine the relationship between propensity for intramolecular cyclisation and intermolecular network formation. We demonstrate a goldilocks zone of maximum reactivity between the sterically hindered and entropically limited regimes with a quantum yield of intramolecular cyclisation that is nearly an order of magnitude higher than the lowest value. As a result of the molecular design of trifunctional macromolecules, their quantum yields can be deconvoluted into the formation of two different cyclic isomers, as rationalised with molecular dynamics simulations. Critically, we visualise our solution-based studies with light-based additive manufacturing. We formulate four photoresists for microprinting, revealing that the precise positioning of functional groups is critical for resist performance, with lower intramolecular quantum yields leading to higher-quality printing in most cases.
Abstract Understanding the intricate relationship between molecular architecture and function underpins most challenges at the forefront of chemical innovation. Bond-forming reactions are particularly influenced by the topology of a chemical structure, both on small molecule scale and in larger macromolecular frameworks. Herein, we elucidate the impact that molecular architecture has on the photo-induced cyclisations of a series of monodisperse macromolecules with defined spacers between photodimerisable moieties, and examine the relationship between propensity for intramolecular cyclisation and intermolecular network formation. We demonstrate a goldilocks zone of maximum reactivity between the sterically hindered and entropically limited regimes with a quantum yield of intramolecular cyclisation that is nearly an order of magnitude higher than the lowest value. As a result of the molecular design of trifunctional macromolecules, their quantum yields can be deconvoluted into the formation of two different cyclic isomers, as rationalised with molecular dynamics simulations. Critically, we visualise our solution-based studies with light-based additive manufacturing. We formulate four photoresists for microprinting, revealing that the precise positioning of functional groups is critical for resist performance, with lower intramolecular quantum yields leading to higher-quality printing in most cases.
Design and Continuous (Re)Processing of Thermally Resilient Poly(Styrene-co-Maleic Maleate)-Based Covalent Adaptable Networks
A. Hernández; T. Maiheu; E. Drockenmuller; D. Montarnal; J.M. Winne; F.E. Du Prez
Chem. Mater., 2024
A. Hernández; T. Maiheu; E. Drockenmuller; D. Montarnal; J.M. Winne; F.E. Du Prez
Chem. Mater., 2024
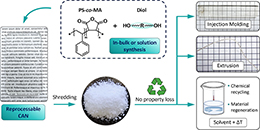 Abstract Cyclic anhydrides, classical monomers for bulk synthetic materials, have recently gained attention in covalent adaptable networks (CANs). They undergo reversible ring-opening with polyols, forming dynamic monoester linkages. This inherent property makes cyclic anhydrides ideal for industrially relevant polymer matrices with CAN-like recycling and reprocessing capabilities. Our study presents a rational design for poly(styrene-co-maleic maleate) monoester (PS-MME)-based polymer networks, achieved by cross-linking commercially available poly(styrene-co-maleic anhydride) (PS-co-MA) with a series of diols. The network synthesis is not only feasible on a large scale and under solvent-free conditions but also allows for the tailoring of the final cross-linking degree and properties by independently tuning the PS-co-MA maleic anhydride content, its chain length, and/or the loading of cross-linker diols used. Previous limitations in the thermal stability of MME-based CANs, stemming from remaining free carboxyl acids in monoester linkages, are circumvented through the dilution of reactive sites in an apolar matrix and switching to a cyclic anhydride ring embedded into the polymer backbone. This robust macromolecular architecture enhances the kinetics and thermodynamics of reversible monoester formation, resulting in PS-MMEs with exceptional thermal resilience.
Abstract Cyclic anhydrides, classical monomers for bulk synthetic materials, have recently gained attention in covalent adaptable networks (CANs). They undergo reversible ring-opening with polyols, forming dynamic monoester linkages. This inherent property makes cyclic anhydrides ideal for industrially relevant polymer matrices with CAN-like recycling and reprocessing capabilities. Our study presents a rational design for poly(styrene-co-maleic maleate) monoester (PS-MME)-based polymer networks, achieved by cross-linking commercially available poly(styrene-co-maleic anhydride) (PS-co-MA) with a series of diols. The network synthesis is not only feasible on a large scale and under solvent-free conditions but also allows for the tailoring of the final cross-linking degree and properties by independently tuning the PS-co-MA maleic anhydride content, its chain length, and/or the loading of cross-linker diols used. Previous limitations in the thermal stability of MME-based CANs, stemming from remaining free carboxyl acids in monoester linkages, are circumvented through the dilution of reactive sites in an apolar matrix and switching to a cyclic anhydride ring embedded into the polymer backbone. This robust macromolecular architecture enhances the kinetics and thermodynamics of reversible monoester formation, resulting in PS-MMEs with exceptional thermal resilience.
Exploring the dual dynamic synergy of transesterification and siloxane exchange in vitrimers
S. Fadlallah, F. Van Lijsebetten, T. Debsharma, V. Scholiers, F. Allais, F.E. Du Prez
Eur. Polym. J., 2024
S. Fadlallah, F. Van Lijsebetten, T. Debsharma, V. Scholiers, F. Allais, F.E. Du Prez
Eur. Polym. J., 2024
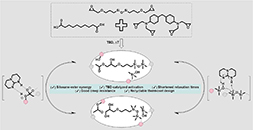 Abstract Despite the benefits of dynamic polymer networks with multiple dynamic bonds, identifying compatible combinations of dynamic chemistries that work synergistically to achieve desirable properties, remains a significant challenge. This work focuses on the potential of utilizing both siloxane and ester dynamic bonds in epoxy-acid cured vitrimers to finetune chemical exchange reactions. We identified to what extent a common basic catalyst (TBD) can simultaneously activate siloxane and ester exchange in the corresponding epoxy-based vitrimers. Our results showed that TBD is not only able to facilitate network formation but also improved the dynamic behavior of the resulting networks dramatically, with an overall exchange rate faster than the sum of the individual exchange chemistries, as shown by stress-relaxation studies. It has been demonstrated that the siloxane and ester dynamic bonds work together in a synergistic way to facilitate topology rearrangement. The relative increase or decrease in mobility of neighboring chains, located around a specific dynamic bond within the polymer network, was investigated in detail by adjusting the concentration of dynamic bonds and catalyst. The herein reported strategy allows the production of dual dynamic polymer networks that exhibit much shorter relaxation times and thus improved (re)processability in comparison to vitrimers with one type of dynamic bond.
Abstract Despite the benefits of dynamic polymer networks with multiple dynamic bonds, identifying compatible combinations of dynamic chemistries that work synergistically to achieve desirable properties, remains a significant challenge. This work focuses on the potential of utilizing both siloxane and ester dynamic bonds in epoxy-acid cured vitrimers to finetune chemical exchange reactions. We identified to what extent a common basic catalyst (TBD) can simultaneously activate siloxane and ester exchange in the corresponding epoxy-based vitrimers. Our results showed that TBD is not only able to facilitate network formation but also improved the dynamic behavior of the resulting networks dramatically, with an overall exchange rate faster than the sum of the individual exchange chemistries, as shown by stress-relaxation studies. It has been demonstrated that the siloxane and ester dynamic bonds work together in a synergistic way to facilitate topology rearrangement. The relative increase or decrease in mobility of neighboring chains, located around a specific dynamic bond within the polymer network, was investigated in detail by adjusting the concentration of dynamic bonds and catalyst. The herein reported strategy allows the production of dual dynamic polymer networks that exhibit much shorter relaxation times and thus improved (re)processability in comparison to vitrimers with one type of dynamic bond.
Polyisoprene-based pressure-sensitive adhesives with dynamic crosslinks
T. Maiheu, J. Debuyck, F. Van Lijsebetten , A. Hernández, F.E. Du Prez
Eur. Polym. J., 2024
T. Maiheu, J. Debuyck, F. Van Lijsebetten , A. Hernández, F.E. Du Prez
Eur. Polym. J., 2024
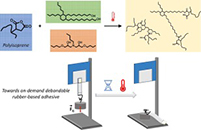 Abstract The reuse of multi-layered materials, which are held together by adhesives, has been identified as a major issue.
In this research, we explored an external catalyst-free transesterification chemistry for thermally triggered, ondemand debondable adhesives that are prepared by reacting readily available (macromolecular) diols through a
one-step process with a rubbery polyisoprene-graft-maleic anhydride matrix. The research starts with a systematic study investigating how the architecture of the diol, the flexibility of its backbone, and the presence of
mildly basic compounds affected the rate and quality of network formation. The dynamic behaviour of the most
promising formulations is then characterised, after which pressure-sensitive adhesive (PSA) specimens have been
made to test the potential for on-demand debonding via temperature-controlled static shear tests. The debonding
process is solely triggered by an increase in temperature (T > 100 ◦C), ensuring the approach is non-destructive
and industrially feasible. The herein reported drop-in method thus provides a starting point for the development
of a new class of rubber-based adhesives that should be able to bond as well as debond various substrates such as
metals and plastics.
Abstract The reuse of multi-layered materials, which are held together by adhesives, has been identified as a major issue.
In this research, we explored an external catalyst-free transesterification chemistry for thermally triggered, ondemand debondable adhesives that are prepared by reacting readily available (macromolecular) diols through a
one-step process with a rubbery polyisoprene-graft-maleic anhydride matrix. The research starts with a systematic study investigating how the architecture of the diol, the flexibility of its backbone, and the presence of
mildly basic compounds affected the rate and quality of network formation. The dynamic behaviour of the most
promising formulations is then characterised, after which pressure-sensitive adhesive (PSA) specimens have been
made to test the potential for on-demand debonding via temperature-controlled static shear tests. The debonding
process is solely triggered by an increase in temperature (T > 100 ◦C), ensuring the approach is non-destructive
and industrially feasible. The herein reported drop-in method thus provides a starting point for the development
of a new class of rubber-based adhesives that should be able to bond as well as debond various substrates such as
metals and plastics.
Thermoswitchable catalysis to inhibit and promote plastic flow in vitrimers
F. Van Lijsebetten; S. Maes; J.M. Winne; F.E. Du Prez
Chem. Sci., 2024
F. Van Lijsebetten; S. Maes; J.M. Winne; F.E. Du Prez
Chem. Sci., 2024
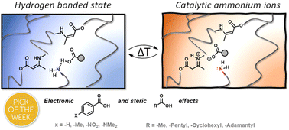 Abstract Acid-base catalysis is a common strategy to induce covalent bond exchanges in dynamic polymer networks. Strong acids or strong bases can promote rapid network rearrangements, and are simultaneously preferred catalysts for chemical reactions where maximum efficiency at the lowest possible temperature is aimed for. However, within the context of dynamic polymer networks, the incorporation of highly active catalysts can negatively affect the longer term application potential. Network dynamicity can diminish through catalyst ageing or quenching and highly active catalysts may prematurely activate bond exchanges, leading to dimensional instability and thus low creep resistance of the polymer networks. Herein, we present several examples where we explicitly explored weak acids (carboxylic acids) as catalysts for dynamic bond exchanges, using vinylogous urethanes (VU) as a well-understood protic acid catalysed vitrimer chemistry. Surprisingly, we have found that the sought-after long-term stability offered by a weak acid does not necessarily bring lower activity at high temperature. In fact, the weak acids show a remarkable thermoswitchable catalytic behaviour, going from an inactive hydrogen bonded state to an active state where the polymer matrix is protonated, with a profound impact on the network reactivity and rheology.
Abstract Acid-base catalysis is a common strategy to induce covalent bond exchanges in dynamic polymer networks. Strong acids or strong bases can promote rapid network rearrangements, and are simultaneously preferred catalysts for chemical reactions where maximum efficiency at the lowest possible temperature is aimed for. However, within the context of dynamic polymer networks, the incorporation of highly active catalysts can negatively affect the longer term application potential. Network dynamicity can diminish through catalyst ageing or quenching and highly active catalysts may prematurely activate bond exchanges, leading to dimensional instability and thus low creep resistance of the polymer networks. Herein, we present several examples where we explicitly explored weak acids (carboxylic acids) as catalysts for dynamic bond exchanges, using vinylogous urethanes (VU) as a well-understood protic acid catalysed vitrimer chemistry. Surprisingly, we have found that the sought-after long-term stability offered by a weak acid does not necessarily bring lower activity at high temperature. In fact, the weak acids show a remarkable thermoswitchable catalytic behaviour, going from an inactive hydrogen bonded state to an active state where the polymer matrix is protonated, with a profound impact on the network reactivity and rheology.
Unprecedented associative exchange in CO2-sourced cyclic S,O-acetal-based covalent adaptable networks
S. Maes; T. Habets; S.M. Fischer; B. Grignard; C. Detrembleur; F.E. Du Prez
Polym. Chem., 2024
S. Maes; T. Habets; S.M. Fischer; B. Grignard; C. Detrembleur; F.E. Du Prez
Polym. Chem., 2024
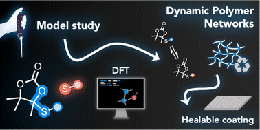 Abstract New dynamic chemistry is nowadays sought to widen the library of accessible covalent adaptable networks (CANs). Here, we investigate the dynamic nature of CO2-sourced cyclic S,O-acetal bonds under unexplored conditions. Model molecule studies were conducted on various compounds and supported by extensive DFT calculations to understand the required conditions for triggering exchange and the underlying reaction mechanisms. This is the first study to report dynamic S,O-acetal bonds with an unprecedented associative exchange mechanism occurring through nucleophilic attack onto a remote function from the exchanged site. Our findings were translated to macromolecular engineering with the successful production of CO2-sourced CANs embedding cyclic S,O-acetal bonds from bifunctional alkylidene cyclic carbonates and polythiols. The polymer properties were tuned by the use of structurally divergent monomers, affording materials with distinct thermal and mechanical properties (e.g. Tg ranging from 2 to 51 °C). Complex relaxation behaviour was recorded by rheology experiments, suggesting concurrent exchange reactions to take place at elevated temperatures. The materials dynamics was leveraged through recycling by compression molding for over five cycles. Furthermore, a proof-of-concept coating application was developed, showcasing damage healing at high temperatures.
Abstract New dynamic chemistry is nowadays sought to widen the library of accessible covalent adaptable networks (CANs). Here, we investigate the dynamic nature of CO2-sourced cyclic S,O-acetal bonds under unexplored conditions. Model molecule studies were conducted on various compounds and supported by extensive DFT calculations to understand the required conditions for triggering exchange and the underlying reaction mechanisms. This is the first study to report dynamic S,O-acetal bonds with an unprecedented associative exchange mechanism occurring through nucleophilic attack onto a remote function from the exchanged site. Our findings were translated to macromolecular engineering with the successful production of CO2-sourced CANs embedding cyclic S,O-acetal bonds from bifunctional alkylidene cyclic carbonates and polythiols. The polymer properties were tuned by the use of structurally divergent monomers, affording materials with distinct thermal and mechanical properties (e.g. Tg ranging from 2 to 51 °C). Complex relaxation behaviour was recorded by rheology experiments, suggesting concurrent exchange reactions to take place at elevated temperatures. The materials dynamics was leveraged through recycling by compression molding for over five cycles. Furthermore, a proof-of-concept coating application was developed, showcasing damage healing at high temperatures.
Aza-Michael Chemistry for PDMS-Based Covalent Adaptable Elastomers: Design and Dual Role of the Silica Filler
L.T. Nguyen; C. Mertens; F.E. Du Prez
Macromol., 2024
L.T. Nguyen; C. Mertens; F.E. Du Prez
Macromol., 2024
Thermomechanical characterisation of reprocessable, siloxane-based, glass-fibre-reinforced vitrimers
V. Amfilochiou, T. Debsharma, I. De Baere, L. Daelemans, F.E. Du Prez, W. Van Paepegem
Compos. B Eng., 2024
V. Amfilochiou, T. Debsharma, I. De Baere, L. Daelemans, F.E. Du Prez, W. Van Paepegem
Compos. B Eng., 2024
Foam-to-Elastomer Recycling of Polyurethane Materials through Incorporation of Dynamic Covalent TAD–Indole Linkages
K. Unal, D. Maes, L. Stricker, K. Lorenz, F.E. Du Prez, L. Imbernon, J.M. Winne
ACS Appl. Polym. Mater., 2024
K. Unal, D. Maes, L. Stricker, K. Lorenz, F.E. Du Prez, L. Imbernon, J.M. Winne
ACS Appl. Polym. Mater., 2024
Vinylogous Urea—Urethane Vitrimers: Accelerating and Inhibiting Network Dynamics through Hydrogen Bonding
S.Engelen, N.D. Dolinski, C. Chen, E. Ghimire, C.A. Lindberg, A.E. Crolais, N. Nitta, J.M. Winne, S.J. Rowan, F.E. Du Prez
Angew. Chem., 2024
S.Engelen, N.D. Dolinski, C. Chen, E. Ghimire, C.A. Lindberg, A.E. Crolais, N. Nitta, J.M. Winne, S.J. Rowan, F.E. Du Prez
Angew. Chem., 2024
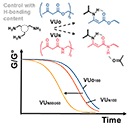 Abstract Vinylogous urethane (VUO) based polymer networks are widely used as catalyst-free vitrimers that show rapid covalent bond exchange at elevated temperatures. In solution, vinylogous ureas (VUN) undergo much faster bond exchange than VUO and are highly dynamic at room temperature. However, this difference in reactivity is not observed in their respective dynamic polymer networks, as VUO and VUN vitrimers prepared herein with very similar macromolecular architectures show comparable stress relaxation and creep behavior. However, by using mixtures of VUO and VUN linkages within the same network, the dynamic reactions can be accelerated by an order of magnitude. The results can be rationalized by the effect of intermolecular hydrogen bonding, which is absent in VUO vitrimers, but is very pronounced for vinylogous urea moieties. At low concentrations of VUN, these hydrogen bonds act as catalysts for covalent bond exchange, while at high concentration, they provide a pervasive vinylogous urea - urethane (VU) network of strong non-covalent interactions, giving rise to phase separation and inhibiting polymer chain dynamics. This offers a straightforward design principle for dynamic polymer materials, showing at the same time the possible additive and synergistic effects of supramolecular and dynamic covalent polymer networks.
Abstract Vinylogous urethane (VUO) based polymer networks are widely used as catalyst-free vitrimers that show rapid covalent bond exchange at elevated temperatures. In solution, vinylogous ureas (VUN) undergo much faster bond exchange than VUO and are highly dynamic at room temperature. However, this difference in reactivity is not observed in their respective dynamic polymer networks, as VUO and VUN vitrimers prepared herein with very similar macromolecular architectures show comparable stress relaxation and creep behavior. However, by using mixtures of VUO and VUN linkages within the same network, the dynamic reactions can be accelerated by an order of magnitude. The results can be rationalized by the effect of intermolecular hydrogen bonding, which is absent in VUO vitrimers, but is very pronounced for vinylogous urea moieties. At low concentrations of VUN, these hydrogen bonds act as catalysts for covalent bond exchange, while at high concentration, they provide a pervasive vinylogous urea - urethane (VU) network of strong non-covalent interactions, giving rise to phase separation and inhibiting polymer chain dynamics. This offers a straightforward design principle for dynamic polymer materials, showing at the same time the possible additive and synergistic effects of supramolecular and dynamic covalent polymer networks.
Telechelic sequence-defined oligoamides: their step-economical synthesis, depolymerization and use in polymer networks
I. De Franceschi, N. Badi, F.E. Du Prez
Chem. Sci., 2024
I. De Franceschi, N. Badi, F.E. Du Prez
Chem. Sci., 2024
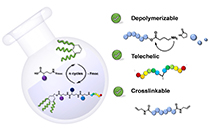 Abstract The application of sequence-defined macromolecules in material science remains largely unexplored due to their challenging, low yielding and time-consuming synthesis. This work first describes a step-economical method for synthesizing unnatural sequence-defined oligoamides through fluorenylmethyloxycarbonyl chemistry. The use of a monodisperse soluble support enables homogeneous reactions at elevated temperature (up to 65 °C), leading to rapid coupling times (<10 min) and improved synthesis protocols. Moreover, a one-pot procedure for the two involved iterative steps is demonstrated via an intermediate quenching step, eliminating the need for in-between purification. The protocol is optimized using γ-aminobutyric acid (GABA) as initial amino acid, and the unique ability of the resulting oligomers to depolymerize, with the formation of cyclic γ-butyrolactame, is evidenced. Furthermore, in order to demonstrate the versatility of the present protocol, a library of 17 unnatural amino acid monomers is synthesized, starting from the readily available GABA-derivative 4-amino-2-hydroxybutanoic acid, and then used to create multifunctional tetramers. Notably, the obtained tetramers show higher thermal stability than a similar thiolactone-based sequence-defined macromolecule, which enables its exploration within a material context.
Abstract The application of sequence-defined macromolecules in material science remains largely unexplored due to their challenging, low yielding and time-consuming synthesis. This work first describes a step-economical method for synthesizing unnatural sequence-defined oligoamides through fluorenylmethyloxycarbonyl chemistry. The use of a monodisperse soluble support enables homogeneous reactions at elevated temperature (up to 65 °C), leading to rapid coupling times (<10 min) and improved synthesis protocols. Moreover, a one-pot procedure for the two involved iterative steps is demonstrated via an intermediate quenching step, eliminating the need for in-between purification. The protocol is optimized using γ-aminobutyric acid (GABA) as initial amino acid, and the unique ability of the resulting oligomers to depolymerize, with the formation of cyclic γ-butyrolactame, is evidenced. Furthermore, in order to demonstrate the versatility of the present protocol, a library of 17 unnatural amino acid monomers is synthesized, starting from the readily available GABA-derivative 4-amino-2-hydroxybutanoic acid, and then used to create multifunctional tetramers. Notably, the obtained tetramers show higher thermal stability than a similar thiolactone-based sequence-defined macromolecule, which enables its exploration within a material context.
β-Amino amide based covalent adaptable networks with high dimensional stability
L. Tan Nguyen, F. Portone, F.E. Du Prez
Polym. Chem., 2024
L. Tan Nguyen, F. Portone, F.E. Du Prez
Polym. Chem., 2024
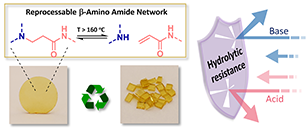 Abstract Herein, we report a scalable synthesis of catalyst-free, covalent adaptable networks (CANs) based on β-amino amides as dynamic linkages. Rheological analysis of their dynamic behaviour shows a remarkably high activation energy of around 300 kJ mol−1. Hence, the obtained elastomers can be (re-)processed at elevated temperatures while possessing high creep resistance in a wide temperature window. Finally, in comparison with the much-studied β-amino ester-based networks, this new generation of CANs incorporating amino amide motifs possess superior hydrolytic resistance under both acidic and basic conditions.
Abstract Herein, we report a scalable synthesis of catalyst-free, covalent adaptable networks (CANs) based on β-amino amides as dynamic linkages. Rheological analysis of their dynamic behaviour shows a remarkably high activation energy of around 300 kJ mol−1. Hence, the obtained elastomers can be (re-)processed at elevated temperatures while possessing high creep resistance in a wide temperature window. Finally, in comparison with the much-studied β-amino ester-based networks, this new generation of CANs incorporating amino amide motifs possess superior hydrolytic resistance under both acidic and basic conditions.
Photo-Crosslinking and Reductive Decrosslinking of Polymethacrylate-Based Copolymers Containing 1,2-Dithiolane Rings
S. Maes, V. Scholiers, F.E. Du Prez
Macromol. Chem. Phys., 2023
S. Maes, V. Scholiers, F.E. Du Prez
Macromol. Chem. Phys., 2023
.png) Abstract This study first describes a straightforward approach for the preparation of polymethacrylate-based networks containing variable amounts of lipoates. For this purpose, butyl methacrylate and a 1,2-dithiolane derivative of hydroxymethylmethacrylate are copolymerized in different ratios by free-radical polymerization with the formation of linear network precursors. After optimizing the copolymerization conditions, the ultraviolet (UV)-induced crosslinking behavior is studied. The latter is possible because of the presence of 1,2-dithiolane moieties, which can be photolytically cleaved and recombined upon UV-irradiation. The obtained networks are analyzed in terms of swelling degree, gel fraction, and rheological behavior. Then, network decrosslinking is achieved by immersing the networks in a reducing environment. Finally, successful preliminary coating experiments are conducted, demonstrating the potential applicability of the studied materials.
Abstract This study first describes a straightforward approach for the preparation of polymethacrylate-based networks containing variable amounts of lipoates. For this purpose, butyl methacrylate and a 1,2-dithiolane derivative of hydroxymethylmethacrylate are copolymerized in different ratios by free-radical polymerization with the formation of linear network precursors. After optimizing the copolymerization conditions, the ultraviolet (UV)-induced crosslinking behavior is studied. The latter is possible because of the presence of 1,2-dithiolane moieties, which can be photolytically cleaved and recombined upon UV-irradiation. The obtained networks are analyzed in terms of swelling degree, gel fraction, and rheological behavior. Then, network decrosslinking is achieved by immersing the networks in a reducing environment. Finally, successful preliminary coating experiments are conducted, demonstrating the potential applicability of the studied materials.
Trialkylsulfonium-Based Reprocessable Polyurethane Thermosets
V. Scholiers, B. Hendriks, S. Maes, T. Debsharma, J.M. Winne, F.E. Du Prez
Macromol., 2023
V. Scholiers, B. Hendriks, S. Maes, T. Debsharma, J.M. Winne, F.E. Du Prez
Macromol., 2023
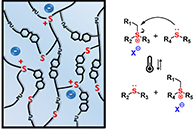 Abstract We report the development of a solvent-free protocol to produce colorless, highly transparent, and glassy polyurethane-based networks containing thioether bonds using commercially available building blocks. These polyurethane networks are converted into reprocessable networks by partial alkylation of the thioether bonds, giving dynamic trialkylsulfonium bonds that are able to exchange via transalkylation at elevated temperatures, thus inducing viscoelastic flow. Reprocessability of the trialkylsulfonium networks was demonstrated for three cycles without significant degradation of material properties. Interestingly, these materials were found to be highly processable at elevated temperatures (∼140 °C) and showed excellent creep suppression up to 100 °C, a combination that is rare among dynamic covalent polymer networks. The suppression of creep can be further controlled by changing the alkylating additive. In addition, as a result of their excellent transparency and high clarity, we investigated their optical properties to assess their potential use in smart coatings and optical devices.
Abstract We report the development of a solvent-free protocol to produce colorless, highly transparent, and glassy polyurethane-based networks containing thioether bonds using commercially available building blocks. These polyurethane networks are converted into reprocessable networks by partial alkylation of the thioether bonds, giving dynamic trialkylsulfonium bonds that are able to exchange via transalkylation at elevated temperatures, thus inducing viscoelastic flow. Reprocessability of the trialkylsulfonium networks was demonstrated for three cycles without significant degradation of material properties. Interestingly, these materials were found to be highly processable at elevated temperatures (∼140 °C) and showed excellent creep suppression up to 100 °C, a combination that is rare among dynamic covalent polymer networks. The suppression of creep can be further controlled by changing the alkylating additive. In addition, as a result of their excellent transparency and high clarity, we investigated their optical properties to assess their potential use in smart coatings and optical devices.
Thermally Responsive Selenide-containing Materials Based on Transalkylation of Selenonium Salts
S. Chen, V. Scholiers, M. Zhang, J. Zhang, J. Zhu, F.E. Du Prez, X. Pan
Angew. Chem. Int. Ed., 2023
S. Chen, V. Scholiers, M. Zhang, J. Zhang, J. Zhu, F.E. Du Prez, X. Pan
Angew. Chem. Int. Ed., 2023
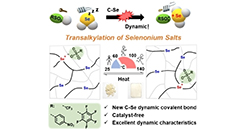 Abstract Covalent adaptable networks (CANs) possess unique properties as a result of their internal dynamic bonds, such as self-healing and reprocessing abilities. In this study, we report a thermally responsive C−Se dynamic covalent chemistry (DCC) that relies on the transalkylation exchange between selenonium salts and selenides, which undergo a fast transalkylation reaction in the absence of any catalyst. Additionally, we demonstrate the presence of a dissociative mechanism in the absence of selenide groups. After incorporation of this DCC into selenide-containing polymer materials, it was observed that the cross-linked networks display varying dynamic exchange rates when using different alkylation reagents, suggesting that the reprocessing capacity of selenide-containing materials can be regulated. Also, by incorporating selenonium salts into polymer materials, we observed that the materials exhibited good healing ability at elevated temperatures as well as excellent solvent resistance at ambient temperature. This novel dynamic covalent chemistry thus provides a straightforward method for the healing and reprocessing of selenide-containing materials.
Abstract Covalent adaptable networks (CANs) possess unique properties as a result of their internal dynamic bonds, such as self-healing and reprocessing abilities. In this study, we report a thermally responsive C−Se dynamic covalent chemistry (DCC) that relies on the transalkylation exchange between selenonium salts and selenides, which undergo a fast transalkylation reaction in the absence of any catalyst. Additionally, we demonstrate the presence of a dissociative mechanism in the absence of selenide groups. After incorporation of this DCC into selenide-containing polymer materials, it was observed that the cross-linked networks display varying dynamic exchange rates when using different alkylation reagents, suggesting that the reprocessing capacity of selenide-containing materials can be regulated. Also, by incorporating selenonium salts into polymer materials, we observed that the materials exhibited good healing ability at elevated temperatures as well as excellent solvent resistance at ambient temperature. This novel dynamic covalent chemistry thus provides a straightforward method for the healing and reprocessing of selenide-containing materials.
Reprocessable Polyurethane Foams Using Acetoacetyl-Formed
Amides
H. Kassem, L. Imbernon, L. Stricker, L. Jonckheere, F.E. Du Prez
Appl. Mater. Interfaces, 2023
H. Kassem, L. Imbernon, L. Stricker, L. Jonckheere, F.E. Du Prez
Appl. Mater. Interfaces, 2023
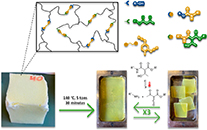 Abstract Like any other thermosetting material, polyurethane foams
(PUFs) contain permanent cross-links that hinder their reprocessability
and make their recyclability a tedious and environmentally unfriendly
process. Herein, we introduce acetoacetyl-formed amides, formed by the
reaction of isocyanates with acetoacetate groups, as dynamic units in the
backbone of PUFs. By extensive variation of the foam composition,
optimum parameters have been found to produce malleable foams above
temperatures of 130 °C, without the requirement of any solvent during
the foaming process. The PU cross-linked material can be compressionmolded
at least three times, giving rise to PU elastomers and thus
maintaining a cross-linked network structure. Characterization of the
original foams shows comparable properties to standard PUFs, for
example, having a density of 32 kg/m3, while they show similar chemical
and thermal properties upon reprocessing to strong PU elastomers,
exhibiting Tg ranging from −42 to −48 °C. This research provides a straightforward method to produce thermally reprocessable
PUFs as a promising pathway to address the recycling issues of end-of-life foams.
Abstract Like any other thermosetting material, polyurethane foams
(PUFs) contain permanent cross-links that hinder their reprocessability
and make their recyclability a tedious and environmentally unfriendly
process. Herein, we introduce acetoacetyl-formed amides, formed by the
reaction of isocyanates with acetoacetate groups, as dynamic units in the
backbone of PUFs. By extensive variation of the foam composition,
optimum parameters have been found to produce malleable foams above
temperatures of 130 °C, without the requirement of any solvent during
the foaming process. The PU cross-linked material can be compressionmolded
at least three times, giving rise to PU elastomers and thus
maintaining a cross-linked network structure. Characterization of the
original foams shows comparable properties to standard PUFs, for
example, having a density of 32 kg/m3, while they show similar chemical
and thermal properties upon reprocessing to strong PU elastomers,
exhibiting Tg ranging from −42 to −48 °C. This research provides a straightforward method to produce thermally reprocessable
PUFs as a promising pathway to address the recycling issues of end-of-life foams.
Enhanced Viscosity Control in Thermosets Derived from Epoxy and Acrylate Monomers Based on Thermoreversible Aza-Michael Chemistry
S. Engelen; F. Van Lijsebetten; R. Aksakal; J.M. Winne; F.E. Du Prez
Macromol., 2023
S. Engelen; F. Van Lijsebetten; R. Aksakal; J.M. Winne; F.E. Du Prez
Macromol., 2023
.jpeg) Abstract Epoxies, epoxy acrylates, and acrylic resins account for a large portion of the thermoset market, yet their inherently highly cross-linked nature prevents them from being reprocessed or recycled. Herein, we present a simple reversible epoxy-based curing strategy that can address these issues. First, an epoxy monomer is ring-opened with ammonia to result in β-hydroxyamines through a straightforward and scalable synthesis that we have demonstrated on multiple examples. The epoxy-derived amines are then cured with bisacrylates, creating dynamic covalent β-amino ester cross-linkages through a thermoreversible aza-Michael reaction. The resulting networks show a very pronounced drop in viscosity in the temperature region of 150–180 °C, which we were able to attribute mainly to significant de-cross-linking of the amine and acrylate moieties, rather than to activation of dynamic ester bond exchanges. Nevertheless, the materials do not fully liquify and retain their structural integrity as a result of the very fast amine-acrylate rebonding kinetics. As a result, cross-linked epoxy-based materials could be obtained with simultaneously enhanced (re)processability at high temperatures and strongly inhibited deformation at lower temperatures (<120 °C).
Abstract Epoxies, epoxy acrylates, and acrylic resins account for a large portion of the thermoset market, yet their inherently highly cross-linked nature prevents them from being reprocessed or recycled. Herein, we present a simple reversible epoxy-based curing strategy that can address these issues. First, an epoxy monomer is ring-opened with ammonia to result in β-hydroxyamines through a straightforward and scalable synthesis that we have demonstrated on multiple examples. The epoxy-derived amines are then cured with bisacrylates, creating dynamic covalent β-amino ester cross-linkages through a thermoreversible aza-Michael reaction. The resulting networks show a very pronounced drop in viscosity in the temperature region of 150–180 °C, which we were able to attribute mainly to significant de-cross-linking of the amine and acrylate moieties, rather than to activation of dynamic ester bond exchanges. Nevertheless, the materials do not fully liquify and retain their structural integrity as a result of the very fast amine-acrylate rebonding kinetics. As a result, cross-linked epoxy-based materials could be obtained with simultaneously enhanced (re)processability at high temperatures and strongly inhibited deformation at lower temperatures (<120 °C).
The Power of Action Plots: Unveiling Reaction Selectivity of Light‐Stabilized Dynamic Covalent Chemistry
S.C. Gauci; F.E. Du Prez; J.O. Holloway; H.A. Houck; C. Barner‐Kowollik
Angew. Chem. Int. Ed., 2023
S.C. Gauci; F.E. Du Prez; J.O. Holloway; H.A. Houck; C. Barner‐Kowollik
Angew. Chem. Int. Ed., 2023
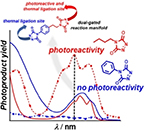 Abstract Exploiting the optimum wavelength of reactivity for efficient photochemical reactions has been well-established based on the development of photochemical action plots. We herein demonstrate the power of such action plots by a remarkable example of the wavelength-resolved photochemistry of two triazolinedione (TAD) substrates, i.e., aliphatic and aromatic substituted, that exhibit near identical absorption spectra yet possess vastly disparate photoreactivity. We present our findings in carefully recorded action plots, from which reaction selectivity is identified. The profound difference in photoreactivity is exploited by designing a ‘hybrid’ bisfunctional TAD molecule, enabling the formation of a dual-gated reaction manifold that demonstrates the exceptional and site-selective (photo)chemical behavior of both TAD substrates within a single small molecule.
Abstract Exploiting the optimum wavelength of reactivity for efficient photochemical reactions has been well-established based on the development of photochemical action plots. We herein demonstrate the power of such action plots by a remarkable example of the wavelength-resolved photochemistry of two triazolinedione (TAD) substrates, i.e., aliphatic and aromatic substituted, that exhibit near identical absorption spectra yet possess vastly disparate photoreactivity. We present our findings in carefully recorded action plots, from which reaction selectivity is identified. The profound difference in photoreactivity is exploited by designing a ‘hybrid’ bisfunctional TAD molecule, enabling the formation of a dual-gated reaction manifold that demonstrates the exceptional and site-selective (photo)chemical behavior of both TAD substrates within a single small molecule.
Sequence-defined antibody-recruiting macromolecules
R. Aksakal, C. Tonneaux, A. Uvyn, M. Fossépré, H. Turgut, N. Badi, M. Surin, B.G. De Geest, F.E. Du Prez
Chem. Sci., 2023
R. Aksakal, C. Tonneaux, A. Uvyn, M. Fossépré, H. Turgut, N. Badi, M. Surin, B.G. De Geest, F.E. Du Prez
Chem. Sci., 2023
 Abstract Antibody-recruiting molecules represent a novel class of therapeutic agents that mediate the recruitment of endogenous antibodies to target cells, leading to their elimination by the immune system. Compared to single-ligand copies, macromolecular scaffolds presenting multiple copies of an antibody-binding ligand offer advantages in terms of increased complex avidity. In this study, we describe the synthesis of sequence-defined macromolecules designed for antibody recruitment, utilising dinitrophenol (DNP) as a model antibody-recruiting motif. The use of discrete macromolecules gives access to varying the spacing between DNP motifs while maintaining the same chain length. This characteristic enables the investigation of structure-dependent binding interactions with anti-DNP antibodies. Through solid-phase thiolactone chemistry, we synthesised a series of oligomers with precisely localised DNP motifs along the backbone and a terminal biotin motif for surface immobilisation. Utilising biolayer interferometry analysis, we observed that oligomers with adjacent DNP motifs exhibited enhanced avidity for anti-DNP antibodies. Molecular modelling provided insights into the structures and dynamics of the various macromolecules, shedding light on the accessibility of the ligands to the antibodies.
Abstract Antibody-recruiting molecules represent a novel class of therapeutic agents that mediate the recruitment of endogenous antibodies to target cells, leading to their elimination by the immune system. Compared to single-ligand copies, macromolecular scaffolds presenting multiple copies of an antibody-binding ligand offer advantages in terms of increased complex avidity. In this study, we describe the synthesis of sequence-defined macromolecules designed for antibody recruitment, utilising dinitrophenol (DNP) as a model antibody-recruiting motif. The use of discrete macromolecules gives access to varying the spacing between DNP motifs while maintaining the same chain length. This characteristic enables the investigation of structure-dependent binding interactions with anti-DNP antibodies. Through solid-phase thiolactone chemistry, we synthesised a series of oligomers with precisely localised DNP motifs along the backbone and a terminal biotin motif for surface immobilisation. Utilising biolayer interferometry analysis, we observed that oligomers with adjacent DNP motifs exhibited enhanced avidity for anti-DNP antibodies. Molecular modelling provided insights into the structures and dynamics of the various macromolecules, shedding light on the accessibility of the ligands to the antibodies.
Epoxy Adhesives with Reversible Hardeners: Controllable Thermal Debonding in Bulk and at Interfaces
F. Van Lijsebetten, T. Maiheu, J.M. Winne, F.E. Du Prez
Adv. Mater., 2023
F. Van Lijsebetten, T. Maiheu, J.M. Winne, F.E. Du Prez
Adv. Mater., 2023
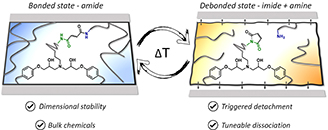 Abstract On-demand adhesive dismantling has the potential to improve multi-material product recycling, but its implementation has been hampered by a critical trade-off between strong bonding and easy debonding. As a result, the temperature range in which these temporary adhesives can be used is relatively limited. Here, we report a new class of dynamic epoxy resins that significantly extends this upper temperature limit and still achieves fast debonding. Specifically, two types of dynamic polyamidoamine curing agents for epoxy hardening were developed, being polysuccinamides (PSA) and polyglutaramides (PGA). As the dynamic debonding/rebonding process of PSA and especially PGA linkages is more thermally demanding and at the same time more thermally robust than previously reported dynamic covalent systems, the resulting materials can be triggered at high temperatures, and at the same time remain bonded over a wide temperature range. The versatility of the PSA and PGA dynamic adhesive curing system was demonstrated in classical bulk adhesive formulations, as well as in dynamic covalent linking to a PSA- or PGA-functionalised surface.
Abstract On-demand adhesive dismantling has the potential to improve multi-material product recycling, but its implementation has been hampered by a critical trade-off between strong bonding and easy debonding. As a result, the temperature range in which these temporary adhesives can be used is relatively limited. Here, we report a new class of dynamic epoxy resins that significantly extends this upper temperature limit and still achieves fast debonding. Specifically, two types of dynamic polyamidoamine curing agents for epoxy hardening were developed, being polysuccinamides (PSA) and polyglutaramides (PGA). As the dynamic debonding/rebonding process of PSA and especially PGA linkages is more thermally demanding and at the same time more thermally robust than previously reported dynamic covalent systems, the resulting materials can be triggered at high temperatures, and at the same time remain bonded over a wide temperature range. The versatility of the PSA and PGA dynamic adhesive curing system was demonstrated in classical bulk adhesive formulations, as well as in dynamic covalent linking to a PSA- or PGA-functionalised surface.
Visible Light-Conjugation with Triazolinediones as a Route to Degradable Poly(ethylene glycol)-Lipids for mRNA Lipid Nanoparticle Formulation
B. Golba, M. Soete, Z. Zhong, N. Sanders, F.E. Du Prez, H.A. Houck, B. de Geest
Angew. Chem., 2023
B. Golba, M. Soete, Z. Zhong, N. Sanders, F.E. Du Prez, H.A. Houck, B. de Geest
Angew. Chem., 2023
 Abstract Polyethylene glycol (PEG) is considered as the gold standard for colloidal stabilization of nanomedicines, yet PEG is non-degradable and lacks functionality on the backbone. Herein, we introduce concomitantly PEG backbone functionality and degradability via a one-step modification with 1,2,4-triazoline-3,5-diones (TAD) under green light. The TAD-PEG conjugates are degradable in aqueous medium under physiological conditions, with the rate of hydrolysis depending on pH and temperature. Subsequently, a PEG-lipid is modified with TAD-derivatives and successfully used for messenger RNA (mRNA) lipid nanoparticle (LNP) delivery, thereby improving mRNA transfection efficiency on multiple cell cultures in vitro. In vivo, in mice, mRNA LNP formulation exhibited a similar tissue distribution as common LNPs, with a slight decrease in transfection efficiency. Our findings pave the road towards the design of degradable, backbone-functionalized PEG for applications in nanomedicine and beyond.
Abstract Polyethylene glycol (PEG) is considered as the gold standard for colloidal stabilization of nanomedicines, yet PEG is non-degradable and lacks functionality on the backbone. Herein, we introduce concomitantly PEG backbone functionality and degradability via a one-step modification with 1,2,4-triazoline-3,5-diones (TAD) under green light. The TAD-PEG conjugates are degradable in aqueous medium under physiological conditions, with the rate of hydrolysis depending on pH and temperature. Subsequently, a PEG-lipid is modified with TAD-derivatives and successfully used for messenger RNA (mRNA) lipid nanoparticle (LNP) delivery, thereby improving mRNA transfection efficiency on multiple cell cultures in vitro. In vivo, in mice, mRNA LNP formulation exhibited a similar tissue distribution as common LNPs, with a slight decrease in transfection efficiency. Our findings pave the road towards the design of degradable, backbone-functionalized PEG for applications in nanomedicine and beyond.
N-Sulfonyl Urethanes to Design Polyurethane Networks with Temperature-Controlled Dynamicity
S. Maes, F. Van Lijsebetten, J.M. Winne, F.E. Du Prez
Macromol., 2023
S. Maes, F. Van Lijsebetten, J.M. Winne, F.E. Du Prez
Macromol., 2023
 Abstract (Re)processing of cross-linked polyurethanes (PUs) is often energy intensive and inefficient since dissociation of urethane linkages at elevated temperatures generates highly reactive isocyanate moieties that can react with a wide range of nucleophiles. In this study, we first show with a small molecule study that the introduction of N-sulfonyl urethane bonds leads to dynamic covalent exchange reactions under much milder conditions compared to regular urethane groups. Then, these exchangeable N-sulfonyl urethane motifs have been introduced, in relatively small amounts (5, 10, and 20%), in a cross-linked PU matrix in an attempt to facilitate plastic flow at lower temperatures. Rheological analysis of the elastomeric dissociative networks revealed an interesting double relaxation behavior, even for temperatures between 150 and 100 °C, which could be described by a Maxwell model with two elements, which can be related to the activated and less activated urethane bonds.
Abstract (Re)processing of cross-linked polyurethanes (PUs) is often energy intensive and inefficient since dissociation of urethane linkages at elevated temperatures generates highly reactive isocyanate moieties that can react with a wide range of nucleophiles. In this study, we first show with a small molecule study that the introduction of N-sulfonyl urethane bonds leads to dynamic covalent exchange reactions under much milder conditions compared to regular urethane groups. Then, these exchangeable N-sulfonyl urethane motifs have been introduced, in relatively small amounts (5, 10, and 20%), in a cross-linked PU matrix in an attempt to facilitate plastic flow at lower temperatures. Rheological analysis of the elastomeric dissociative networks revealed an interesting double relaxation behavior, even for temperatures between 150 and 100 °C, which could be described by a Maxwell model with two elements, which can be related to the activated and less activated urethane bonds.
Resorcinol‐Derived Vitrimers and Their Flax Fiber‐Reinforced Composites Based on Fast Siloxane Exchange
T. Debsharma; S. Engelen; I. De Baere; W. Van Paepegem; F.E. Du Prez
Macromol. Rapid Commun., 2023
T. Debsharma; S. Engelen; I. De Baere; W. Van Paepegem; F.E. Du Prez
Macromol. Rapid Commun., 2023
 Abstract Natural fiber-reinforced composites are gaining increased interest for their significantly reduced carbon footprint compared to conventional glass or carbon fiber-based counterparts. In this study, natural fibers are used in a resorcinol-based epoxy resin that is thermally reshapable at higher temperatures (>180 °C) by using fast exchanging siloxane bonds, catalyzed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene. Stress relaxation times of only about 6 s at 220 °C can be reached. A resorcinol-based epoxy compound is selected because it can be derived from cellulose, opening ways for more sustainable and reshapable composite materials. In a last step of the research, the low viscosity vitrimer formulation (<200 mPa s) is applied to make a flax fiber-reinforced composite using an industrially relevant vacuum-assisted resin infusion process. A section of this composite is successfully reshaped, which allows for envisioning a second life for natural fiber-reinforced composites.
Abstract Natural fiber-reinforced composites are gaining increased interest for their significantly reduced carbon footprint compared to conventional glass or carbon fiber-based counterparts. In this study, natural fibers are used in a resorcinol-based epoxy resin that is thermally reshapable at higher temperatures (>180 °C) by using fast exchanging siloxane bonds, catalyzed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene. Stress relaxation times of only about 6 s at 220 °C can be reached. A resorcinol-based epoxy compound is selected because it can be derived from cellulose, opening ways for more sustainable and reshapable composite materials. In a last step of the research, the low viscosity vitrimer formulation (<200 mPa s) is applied to make a flax fiber-reinforced composite using an industrially relevant vacuum-assisted resin infusion process. A section of this composite is successfully reshaped, which allows for envisioning a second life for natural fiber-reinforced composites.
Regioselective photocycloaddition for light-stabilised dynamic materials design
A.J. Ghielmetti; C. Barner-Kowollik; F.E. Du Prez; H.A. Houck
Polym. Chem., 2023
A.J. Ghielmetti; C. Barner-Kowollik; F.E. Du Prez; H.A. Houck
Polym. Chem., 2023
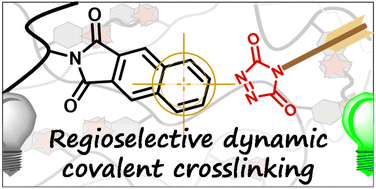 Abstract The exact molecular network connectivity in dynamic polymer networks greatly impacts material properties and reprocessing conditions. While endo/exo stereoisomer effects of thermoreversible cycloaddition reactions (e.g. furan/maleimide) are well known to influence the overall decrosslinking kinetics of dynamic polymer networks, the impact of regioisomerism is often overlooked. Herein, we highlight the importance of interchanging regioisomer fractions in light-stabilised dynamic materials (LSDMs). Specifically, the photo-driven [4 + 2] cycloaddition of substituted naphthalenes with triazolinediones (TADs) is demonstrated to continuously equilibrate under green light, resulting in an enrichment in one of two regioisomers and effectively changing the overall cycloreversion kinetics. To bypass implications of this chemically inhomogeneous nature of the crosslinks when embedded in a macromolecular gel, a library of naphthalene reaction partners is screened to develop a completely regioselective TAD/naphthalene system. Additionally, naphthalene substitution allowed for a fine control over the cycloreversion kinetics with debonding half-life times ranging from 9 to 260 h at ambient temperature.
Abstract The exact molecular network connectivity in dynamic polymer networks greatly impacts material properties and reprocessing conditions. While endo/exo stereoisomer effects of thermoreversible cycloaddition reactions (e.g. furan/maleimide) are well known to influence the overall decrosslinking kinetics of dynamic polymer networks, the impact of regioisomerism is often overlooked. Herein, we highlight the importance of interchanging regioisomer fractions in light-stabilised dynamic materials (LSDMs). Specifically, the photo-driven [4 + 2] cycloaddition of substituted naphthalenes with triazolinediones (TADs) is demonstrated to continuously equilibrate under green light, resulting in an enrichment in one of two regioisomers and effectively changing the overall cycloreversion kinetics. To bypass implications of this chemically inhomogeneous nature of the crosslinks when embedded in a macromolecular gel, a library of naphthalene reaction partners is screened to develop a completely regioselective TAD/naphthalene system. Additionally, naphthalene substitution allowed for a fine control over the cycloreversion kinetics with debonding half-life times ranging from 9 to 260 h at ambient temperature.
Reading Information Stored in Synthetic Macromolecules
M. Soete, C. Mertens, N. Badi, F.E. Du Prez
J. Am. Chem. Soc., 2022
M. Soete, C. Mertens, N. Badi, F.E. Du Prez
J. Am. Chem. Soc., 2022
 Abstract The storage of information in synthetic (macro)molecules provides an attractive alternative for current archival storage media, and the advancements made within this area have prompted the investigation of such molecules for numerous other applications (e.g., anti-counterfeiting tags, steganography). While different strategies have been described for storing information at the molecular level, this Perspective aims to provide a critical overview of the most prominent approaches that can be utilized for retrieving the encoded information. The major part will focus on the sequence determination of synthetic macromolecules, wherein information is stored by the precise arrangement of constituting monomers, with an emphasis on chemically aided strategies, (tandem) mass spectrometry, and nanopore sensing. In addition, recent progress in utilizing (mixtures of) small molecules for information storage will be discussed. Finally, the closing remarks aim to highlight which strategy we believe is the most suitable for a series of specific applications, and will also touch upon the future research avenues that can be pursued for reading (macro)molecular information.
Abstract The storage of information in synthetic (macro)molecules provides an attractive alternative for current archival storage media, and the advancements made within this area have prompted the investigation of such molecules for numerous other applications (e.g., anti-counterfeiting tags, steganography). While different strategies have been described for storing information at the molecular level, this Perspective aims to provide a critical overview of the most prominent approaches that can be utilized for retrieving the encoded information. The major part will focus on the sequence determination of synthetic macromolecules, wherein information is stored by the precise arrangement of constituting monomers, with an emphasis on chemically aided strategies, (tandem) mass spectrometry, and nanopore sensing. In addition, recent progress in utilizing (mixtures of) small molecules for information storage will be discussed. Finally, the closing remarks aim to highlight which strategy we believe is the most suitable for a series of specific applications, and will also touch upon the future research avenues that can be pursued for reading (macro)molecular information.
Ring-Opening Metathesis Polymerization for the Synthesis of Terpenoid-Based Pressure-Sensitive Adhesives
S. Engelen, M. Droesbeke, R. Aksakal, F.E. Du Prez
ACS Macro Lett., 2022
S. Engelen, M. Droesbeke, R. Aksakal, F.E. Du Prez
ACS Macro Lett., 2022
 Abstract Pressure-sensitive adhesives (PSAs) made from norbornene-functionalized terpenoid-based monomers are reported as a possible alternative to the conventional petrochemically based PSAs. For this, tetrahydrogeranyl, menthyl, and isobornyl norbornenate monomers, with a renewable carbon content up to 72%, are synthesized and copolymerized via ring-opening metathesis polymerization (ROMP) with cyclooctadiene and 5-norbornene-2-carboxylic acid. ROMP enables a much faster and controlled polymerization process in comparison to free radical polymerization techniques when targeting high molecular weights and therefore unlocks a potential to design a unique class of PSA materials. The moduli at bonding and debonding frequencies of the obtained PSAs are plotted in the Chang classification system and are used to predict their adhesive performance. Tack and peel measurements indicate that the terpenoid-based norbornenate formulations show similar adhesive properties in comparison to the previously investigated acrylic counterparts.
Abstract Pressure-sensitive adhesives (PSAs) made from norbornene-functionalized terpenoid-based monomers are reported as a possible alternative to the conventional petrochemically based PSAs. For this, tetrahydrogeranyl, menthyl, and isobornyl norbornenate monomers, with a renewable carbon content up to 72%, are synthesized and copolymerized via ring-opening metathesis polymerization (ROMP) with cyclooctadiene and 5-norbornene-2-carboxylic acid. ROMP enables a much faster and controlled polymerization process in comparison to free radical polymerization techniques when targeting high molecular weights and therefore unlocks a potential to design a unique class of PSA materials. The moduli at bonding and debonding frequencies of the obtained PSAs are plotted in the Chang classification system and are used to predict their adhesive performance. Tack and peel measurements indicate that the terpenoid-based norbornenate formulations show similar adhesive properties in comparison to the previously investigated acrylic counterparts.
A Highly Dynamic Covalent Polymer Network without Creep: Mission Impossible?
F. Van Lijsebetten, T. Debsharma, J.M. Winne, F.E. Du Prez
Angew. Chem., 2022
F. Van Lijsebetten, T. Debsharma, J.M. Winne, F.E. Du Prez
Angew. Chem., 2022
.jpg) Abstract Dynamic covalent polymer networks provide an interesting solution to the challenging recyclability of thermosets and elastomers. One of the remaining design constraints, however, is balancing thermal reprocessability in the form of material flow with dimensional stability during use. As a result, many chemistries are being investigated in order to improve bond reactivity control and material robustness. This Minireview highlights a number of promising concepts, with a particular emphasis on disconnecting chemical reactivity in low and high temperature regimes to obtain creep resistant, yet highly dynamic polymer networks. In addition, we will highlight the impact of sharp reactivity changes when applying extrapolation-based approaches during rheological analysis. As a result, we are confident that abandoning the myth of “permanent” reactivity will aid in the development of sustainable polymeric materials that can truly combine the benefits of thermoplastic and thermoset behaviour.
Abstract Dynamic covalent polymer networks provide an interesting solution to the challenging recyclability of thermosets and elastomers. One of the remaining design constraints, however, is balancing thermal reprocessability in the form of material flow with dimensional stability during use. As a result, many chemistries are being investigated in order to improve bond reactivity control and material robustness. This Minireview highlights a number of promising concepts, with a particular emphasis on disconnecting chemical reactivity in low and high temperature regimes to obtain creep resistant, yet highly dynamic polymer networks. In addition, we will highlight the impact of sharp reactivity changes when applying extrapolation-based approaches during rheological analysis. As a result, we are confident that abandoning the myth of “permanent” reactivity will aid in the development of sustainable polymeric materials that can truly combine the benefits of thermoplastic and thermoset behaviour.
Bio-based, creep-resistant covalent adaptable networks based on β-amino ester chemistry
L. Stricker, C. Taplan, F.E. Du Prez
ACS Sustainable Chem. Eng., 2022
L. Stricker, C. Taplan, F.E. Du Prez
ACS Sustainable Chem. Eng., 2022
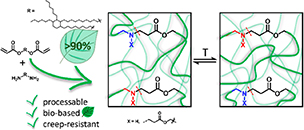 Abstract For this study, creep-resistant covalent adaptable networks were prepared by making use of the reversible β-amino ester chemical platform and starting from biobased raw materials with an overall biobased carbon content of >90%. The investigated materials were synthesized with different cross-linking densities in a solvent-free fashion. The applied building blocks consisted of an easily obtained acrylate, based on a biobased diol, and a commercially available biobased multifunctional amine. Following their synthesis, the materials were investigated with regard to their thermal, thermomechanical, and rheological properties, which were preserved after up to three reprocessing cycles. Moreover, the obtained elastomers showed high thermal stability, in combination with good reprocessability at 180 °C and excellent creep resistance at elevated temperatures of up to 120 °C.
Abstract For this study, creep-resistant covalent adaptable networks were prepared by making use of the reversible β-amino ester chemical platform and starting from biobased raw materials with an overall biobased carbon content of >90%. The investigated materials were synthesized with different cross-linking densities in a solvent-free fashion. The applied building blocks consisted of an easily obtained acrylate, based on a biobased diol, and a commercially available biobased multifunctional amine. Following their synthesis, the materials were investigated with regard to their thermal, thermomechanical, and rheological properties, which were preserved after up to three reprocessing cycles. Moreover, the obtained elastomers showed high thermal stability, in combination with good reprocessability at 180 °C and excellent creep resistance at elevated temperatures of up to 120 °C.
Characterising Different Molecular Landscapes in Dynamic Covalent Networks
F. Van Lijsebetten, K. De Bruycker, E. Van Ruymbeke, J.M. Winne, F.E. Du Prez
Chem. Sci., 2022
F. Van Lijsebetten, K. De Bruycker, E. Van Ruymbeke, J.M. Winne, F.E. Du Prez
Chem. Sci., 2022
.jpg) Abstract Dynamic covalent networks present a unique opportunity to exert molecular-level control on macroscopic material properties, by linking their thermal behaviour to the thermodynamics and kinetics of the underlying chemistry. Yet, existing methods do not allow for the extraction and analysis of the influence of local differences in chemical reactivity caused by available reactants, catalysts, or additives. In this context, we present a rheological paradigm that allows us to correlate the composition of a reactive polymer segment to a faster or slower rate of network rearrangement. We discovered that a generalised Maxwell model could separate and quantify the dynamic behaviour of each type of reactive segment individually, which was crucial to fully comprehend the mechanics of the final material. More specifically, Eyring and Van 't Hoff analysis were used to relate possible bond catalysis and dissociation to structural changes by combining statistical modelling with rheology measurements. As a result, precise viscosity changes could be measured, allowing for accurate comparison of various dynamic covalent network materials, including vitrimers and dissociative networks.
Abstract Dynamic covalent networks present a unique opportunity to exert molecular-level control on macroscopic material properties, by linking their thermal behaviour to the thermodynamics and kinetics of the underlying chemistry. Yet, existing methods do not allow for the extraction and analysis of the influence of local differences in chemical reactivity caused by available reactants, catalysts, or additives. In this context, we present a rheological paradigm that allows us to correlate the composition of a reactive polymer segment to a faster or slower rate of network rearrangement. We discovered that a generalised Maxwell model could separate and quantify the dynamic behaviour of each type of reactive segment individually, which was crucial to fully comprehend the mechanics of the final material. More specifically, Eyring and Van 't Hoff analysis were used to relate possible bond catalysis and dissociation to structural changes by combining statistical modelling with rheology measurements. As a result, precise viscosity changes could be measured, allowing for accurate comparison of various dynamic covalent network materials, including vitrimers and dissociative networks.
Uniform Soluble Support for the large-scale synthesis of sequence-defined macromolecules
I. De Franceschi, C. Mertens, N. Badi, F.E. Du Prez
Polym. Chem., 2022
I. De Franceschi, C. Mertens, N. Badi, F.E. Du Prez
Polym. Chem., 2022
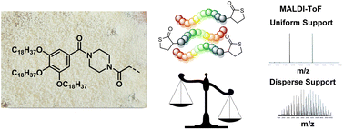 Abstract Herein, a monodisperse soluble support is explored and used as an effective tool for the large-scale, liquid-phase synthesis of sequence-defined macromolecules. This support, based on a benzyl derivative with three long hydrophobic alkyl chains, combines the advantages of solid-phase and soluble support- based synthesis by allowing a straightforward workup after each coupling step and a direct characterisation of the intermediates, without prior cleavage from the support. While the soluble support is used for the multi-gram synthesis of thiolactone-based sequence-defined macromolecules, it can be applied for numerous other synthetic strategies too. Additionally, as it is uniform, it leads to a single peak in mass spectrometry and thus not to a molecular weight distribution as typically observed for standard polymeric soluble supports, thus facilitating the characterisation. To demonstrate the potential for the synthesis of sequence-defined macromolecules on a scale that is sufficient for their introduction in bulk material synthesis, a hexamer is prepared on a 14 g scale. Furthermore, two different cleavable linkers have been attached to the uniform soluble support, hence emphasizing the versatility of this strategy.
Abstract Herein, a monodisperse soluble support is explored and used as an effective tool for the large-scale, liquid-phase synthesis of sequence-defined macromolecules. This support, based on a benzyl derivative with three long hydrophobic alkyl chains, combines the advantages of solid-phase and soluble support- based synthesis by allowing a straightforward workup after each coupling step and a direct characterisation of the intermediates, without prior cleavage from the support. While the soluble support is used for the multi-gram synthesis of thiolactone-based sequence-defined macromolecules, it can be applied for numerous other synthetic strategies too. Additionally, as it is uniform, it leads to a single peak in mass spectrometry and thus not to a molecular weight distribution as typically observed for standard polymeric soluble supports, thus facilitating the characterisation. To demonstrate the potential for the synthesis of sequence-defined macromolecules on a scale that is sufficient for their introduction in bulk material synthesis, a hexamer is prepared on a 14 g scale. Furthermore, two different cleavable linkers have been attached to the uniform soluble support, hence emphasizing the versatility of this strategy.
Recyclable vitrimer epoxy coatings for durable protection
F. Van Lijsebetten, S. Engelen, E. Bauters, W. Van Vooren, M.M.J. Smulders, F.E. Du Prez
Eur. Polym. J., 2022
F. Van Lijsebetten, S. Engelen, E. Bauters, W. Van Vooren, M.M.J. Smulders, F.E. Du Prez
Eur. Polym. J., 2022
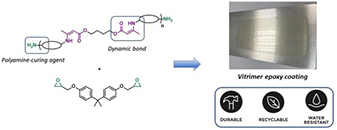 Abstract In this research, the synthesis and application of vitrimer epoxy coatings for long-term corrosion protection of metal surfaces are described. To that end, a polyamine curing agent based on vinylogous urethanes (VUs) was developed to add repairability, recyclability and recoatability to thermosetting materials. Thin films with excellent resistance to water penetration and damage could be obtained by combining the dynamic hardener with existing epoxy resins, resulting in good barrier properties. Various formulations were investigated yielding transparent coatings with durable corrosion protection, even after being scratched, exposed to saltwater, and subjected to steam condensation. Furthermore, stress-relaxation experiments revealed sufficient material flow at 150 °C, which could be significantly slowed down below 100 °C. Optical microscopy was used to demonstrate the healing ability of the vitrimer coatings, resulting in the restoration of barrier properties after scratching. The dynamic properties of the epoxy resin were used to recoat an aluminium plate as a proof of concept, demonstrating that the thin layer could be adhered to a surface again in its cured state. As a result, the service life of epoxy coatings and protected metals could be extended while maintaining excellent mechanical properties, opening the door to a wide range of potential applications such as marine coatings and concrete bonding.
Abstract In this research, the synthesis and application of vitrimer epoxy coatings for long-term corrosion protection of metal surfaces are described. To that end, a polyamine curing agent based on vinylogous urethanes (VUs) was developed to add repairability, recyclability and recoatability to thermosetting materials. Thin films with excellent resistance to water penetration and damage could be obtained by combining the dynamic hardener with existing epoxy resins, resulting in good barrier properties. Various formulations were investigated yielding transparent coatings with durable corrosion protection, even after being scratched, exposed to saltwater, and subjected to steam condensation. Furthermore, stress-relaxation experiments revealed sufficient material flow at 150 °C, which could be significantly slowed down below 100 °C. Optical microscopy was used to demonstrate the healing ability of the vitrimer coatings, resulting in the restoration of barrier properties after scratching. The dynamic properties of the epoxy resin were used to recoat an aluminium plate as a proof of concept, demonstrating that the thin layer could be adhered to a surface again in its cured state. As a result, the service life of epoxy coatings and protected metals could be extended while maintaining excellent mechanical properties, opening the door to a wide range of potential applications such as marine coatings and concrete bonding.
Discrete, self-immolative N-substituted oligourethanes and their use as molecular tags
M. Soete; J. Van Hoorde; F.E.Du Prez
Polym. Chem., 2022
M. Soete; J. Van Hoorde; F.E.Du Prez
Polym. Chem., 2022
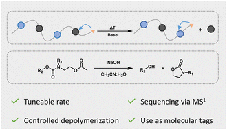 Abstract Here, we first report a straightforward solution-phase approach for the synthesis of N-substituted oligourethanes. Using this protocol, discrete oligomers could be rapidly obtained on a multigram scale by solely utilizing liquid/liquid extractions, avoiding the need for excessive purification steps or the requirement for a solid or soluble support. In addition, structure elucidation of the obtained oligourethanes with the help of ESI- or LC-MS analysis was achieved by relying on the controlled depolymerization of these oligomers. Essential to this process is the presence of the terminal hydroxy moiety that can participate in an intramolecular cyclization. This intramolecular reaction was studied with a series of model compounds in order to determine the parameters that can be used to either enhance or inhibit its rate. In a last step of the study, the applicability of these chemically sequenceable macromolecules as molecular tags was demonstrated by their incorporation in a crosslinked polyurethane material. This study, hence, opens new avenues for the utilization of sequence-defined macromolecules for anti-counterfeiting purposes.
Abstract Here, we first report a straightforward solution-phase approach for the synthesis of N-substituted oligourethanes. Using this protocol, discrete oligomers could be rapidly obtained on a multigram scale by solely utilizing liquid/liquid extractions, avoiding the need for excessive purification steps or the requirement for a solid or soluble support. In addition, structure elucidation of the obtained oligourethanes with the help of ESI- or LC-MS analysis was achieved by relying on the controlled depolymerization of these oligomers. Essential to this process is the presence of the terminal hydroxy moiety that can participate in an intramolecular cyclization. This intramolecular reaction was studied with a series of model compounds in order to determine the parameters that can be used to either enhance or inhibit its rate. In a last step of the study, the applicability of these chemically sequenceable macromolecules as molecular tags was demonstrated by their incorporation in a crosslinked polyurethane material. This study, hence, opens new avenues for the utilization of sequence-defined macromolecules for anti-counterfeiting purposes.
Masked Primary Amines for a Controlled Plastic Flow of Vitrimers
F. Van Lijsebetten, K. De Bruycker, J.M. Winne, F.E. Du Prez
ACS Macro Lett., 2022
F. Van Lijsebetten, K. De Bruycker, J.M. Winne, F.E. Du Prez
ACS Macro Lett., 2022
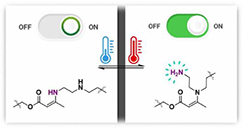 Abstract We present a simple method for increasing the reprocessability of vinylogous urethane (VU) vitrimers while decreasing the possibility of creep deformation at lower temperatures. In particular, varying amounts of triethylenetetramine were added as a comonomer to the curing VU formulation to ensure that all of the primary amines reacted to form enaminone cross-links, resulting in a network without reactive primary amine chain-ends. As a result, transamination was significantly slowed down because secondary amines are much less reactive to VU exchange. On the other hand, at higher temperatures, pendent primary amines can be released via a dynamic, endothermic exchange with a nearby less-reactive secondary amine, thereby (re)activating material flow. As a result, ambivalent viscoelastic behavior could be achieved without depolymerization by dynamically releasing pendent primary amines from vinylogous urethane polymer chains. Through careful comonomer selection, VU vitrimers with low viscosity at processing temperatures and at the same time high viscosity at service temperatures could be prepared without the use of catalysts or additives, leveraging the synergistic effects of mildly reactive functionalities through neighboring group participation.
Abstract We present a simple method for increasing the reprocessability of vinylogous urethane (VU) vitrimers while decreasing the possibility of creep deformation at lower temperatures. In particular, varying amounts of triethylenetetramine were added as a comonomer to the curing VU formulation to ensure that all of the primary amines reacted to form enaminone cross-links, resulting in a network without reactive primary amine chain-ends. As a result, transamination was significantly slowed down because secondary amines are much less reactive to VU exchange. On the other hand, at higher temperatures, pendent primary amines can be released via a dynamic, endothermic exchange with a nearby less-reactive secondary amine, thereby (re)activating material flow. As a result, ambivalent viscoelastic behavior could be achieved without depolymerization by dynamically releasing pendent primary amines from vinylogous urethane polymer chains. Through careful comonomer selection, VU vitrimers with low viscosity at processing temperatures and at the same time high viscosity at service temperatures could be prepared without the use of catalysts or additives, leveraging the synergistic effects of mildly reactive functionalities through neighboring group participation.
Fast Dynamic Siloxane Exchange Mechanism for Reshapable Vitrimer Composites
T. Debsharma, V. Amfilochiou, A.A. Wroblewska, I. De Baere, W. Van Paepegem, F.E. Du Prez
J. Am. Chem. Soc., 2022
T. Debsharma, V. Amfilochiou, A.A. Wroblewska, I. De Baere, W. Van Paepegem, F.E. Du Prez
J. Am. Chem. Soc., 2022
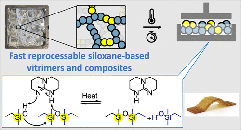 Abstract To develop siloxane-containing vitrimers with fast dynamic characteristics, different mechanistic pathways have been investigated using a range of catalysts. In particular, one siloxane exchange pathway has been found to show a fast dynamic behavior in a useful temperature range (180–220 °C) for its application in vitrimers. The mechanism is found to involve 1,5,7-triazabicyclo [4.4.0] dec-5-ene (TBD) as an organic catalyst in the presence of hydroxyl groups. Using this new mechanistic approach, vitrimers with ultrafast stress-relaxation characteristics (relaxation times below 10 s) have been prepared with a readily available epoxy resin and siloxane-amine hardener. Subsequently, the low viscosity siloxane-containing vitrimer resin enabled the preparation of glass fiber-reinforced vitrimer composites using an industrially relevant vacuum-assisted resin infusion technique. The resulting composite was successfully thermoformed into a new shape, which makes it possible to envision a second life for such highly engineered materials.
Abstract To develop siloxane-containing vitrimers with fast dynamic characteristics, different mechanistic pathways have been investigated using a range of catalysts. In particular, one siloxane exchange pathway has been found to show a fast dynamic behavior in a useful temperature range (180–220 °C) for its application in vitrimers. The mechanism is found to involve 1,5,7-triazabicyclo [4.4.0] dec-5-ene (TBD) as an organic catalyst in the presence of hydroxyl groups. Using this new mechanistic approach, vitrimers with ultrafast stress-relaxation characteristics (relaxation times below 10 s) have been prepared with a readily available epoxy resin and siloxane-amine hardener. Subsequently, the low viscosity siloxane-containing vitrimer resin enabled the preparation of glass fiber-reinforced vitrimer composites using an industrially relevant vacuum-assisted resin infusion technique. The resulting composite was successfully thermoformed into a new shape, which makes it possible to envision a second life for such highly engineered materials.
Force–reversible chemical reaction at ambient temperature for designing toughened dynamic covalent polymer networks
M. Du, H.A. Houck, Q. Yin, Y. Xu, Y. Huang, Y. Lan, L. Yang, F.E. Du Prez, G.Chang
Nat. Commun., 2022
M. Du, H.A. Houck, Q. Yin, Y. Xu, Y. Huang, Y. Lan, L. Yang, F.E. Du Prez, G.Chang
Nat. Commun., 2022
 Abstract Force-reversible C-N bonds, resulting from the click chemistry reaction between triazolinedione (TAD) and indole derivatives, offer exciting opportunities for molecular-level engineering to design materials that respond to mechanical loads. Here, we displayed that TAD-indole adducts, acting as crosslink points in dry-state covalently crosslinked polymers, enable materials to display reversible stress-responsiveness in real time already at ambient temperature. Whereas the exergonic TAD-indole reaction results in the formation of bench-stable adducts, they were shown to dissociate at ambient temperature when embedded in a polymer network and subjected to a stretching force to recover the original products. Moreover, the nascent TAD moiety can spontaneously and immediately be recombined after dissociation with an indole reaction partners at ambient temperature, thus allowing for the adjustment of the polymer segment conformation and the maintenance of the network integrity by force-reversible behaviors. Overall, our strategy represents a general method to create toughened covalently crosslinked polymer materials with simultaneous enhancement of mechanical strength and ductility, which is quite challenging to achieve by conventional chemical methods.
Abstract Force-reversible C-N bonds, resulting from the click chemistry reaction between triazolinedione (TAD) and indole derivatives, offer exciting opportunities for molecular-level engineering to design materials that respond to mechanical loads. Here, we displayed that TAD-indole adducts, acting as crosslink points in dry-state covalently crosslinked polymers, enable materials to display reversible stress-responsiveness in real time already at ambient temperature. Whereas the exergonic TAD-indole reaction results in the formation of bench-stable adducts, they were shown to dissociate at ambient temperature when embedded in a polymer network and subjected to a stretching force to recover the original products. Moreover, the nascent TAD moiety can spontaneously and immediately be recombined after dissociation with an indole reaction partners at ambient temperature, thus allowing for the adjustment of the polymer segment conformation and the maintenance of the network integrity by force-reversible behaviors. Overall, our strategy represents a general method to create toughened covalently crosslinked polymer materials with simultaneous enhancement of mechanical strength and ductility, which is quite challenging to achieve by conventional chemical methods.
Reversible Transformations of Polymer Topologies through Visible Light and Darkness
E. Liarou, H.A. Houck, F.E. Du Prez
J. Am. Chem. Soc., 2022
E. Liarou, H.A. Houck, F.E. Du Prez
J. Am. Chem. Soc., 2022
 Abstract A fundamentally important characteristic of a macromolecule is its shape. Herein, visible light and darkness are used as the only stimuli to reversibly alter the topology of well-defined polymers in a one-pot procedure. For this, linear naphthalene-containing polyacrylates are used as scaffolds for the visible light-induced cycloaddition with various substituted triazolinediones (i.e., butyl, stearyl, perfluoro, and polymeric), resulting in differently shaped graft polymers, including brushes and combs. The thus-formed cycloadduct linkages dissociate in the dark, resulting in the regeneration of the parent linear polymer at ambient temperature, establishing a dual-topology transformation by only switching green light on and off. By applying different temperatures during the cycloreversion process, the dissociation rate of the cycloadducts can be tuned in a facile manner, thus allowing for time control over the regeneration of the parent polymer. By engineering a polymer that consists of differently substituted naphthalenes at the chain ends and on the side chains, the inherently different cycloreversion rates of the formed cycloadducts are leveraged to achieve in situ multi-topology transformations without external stimuli. The shape transformations have been repeated up to 4 times sequentially in one pot without the need of any purification.
Abstract A fundamentally important characteristic of a macromolecule is its shape. Herein, visible light and darkness are used as the only stimuli to reversibly alter the topology of well-defined polymers in a one-pot procedure. For this, linear naphthalene-containing polyacrylates are used as scaffolds for the visible light-induced cycloaddition with various substituted triazolinediones (i.e., butyl, stearyl, perfluoro, and polymeric), resulting in differently shaped graft polymers, including brushes and combs. The thus-formed cycloadduct linkages dissociate in the dark, resulting in the regeneration of the parent linear polymer at ambient temperature, establishing a dual-topology transformation by only switching green light on and off. By applying different temperatures during the cycloreversion process, the dissociation rate of the cycloadducts can be tuned in a facile manner, thus allowing for time control over the regeneration of the parent polymer. By engineering a polymer that consists of differently substituted naphthalenes at the chain ends and on the side chains, the inherently different cycloreversion rates of the formed cycloadducts are leveraged to achieve in situ multi-topology transformations without external stimuli. The shape transformations have been repeated up to 4 times sequentially in one pot without the need of any purification.
Sequence-Defined Mikto-Arm Star-Shaped Macromolecules
M.A. Reith, I. De Franceschi, M. Soete, N. Badi, R. Aksakal, F.E. Du Prez
J. Am. Chem. Soc, 2022
M.A. Reith, I. De Franceschi, M. Soete, N. Badi, R. Aksakal, F.E. Du Prez
J. Am. Chem. Soc, 2022
 Abstract The synthesis of sequence-defined, discrete star-shaped macromolecules is a major challenge due to the lack of straightforward and versatile approaches. Here, a robust strategy is proposed that allows not only the preparation of sequence-defined mikto-arm star-shaped macromolecules but also the synthesis of a series of unprecedented discrete, multifunctional complex architectures with molar masses above 11 kDa. The iterative approach reported makes use of readily available building blocks and results in asymmetrically branched macromolecules with high purity and yields, which is showcased with monodisperse mikto-arm three-, four-, and five-arm star-shaped structures that were all characterized via LC–MS, MALDI-ToF, and NMR. This effective strategy drastically improves upon synthetic abilities of polymer chemists by enabling simultaneously sequence definition, precision insertion of branching points, as well as the orthogonal end-group functionalization of complex polymeric architectures. The presented approach, which can be translated to different platforms such as peptides and peptoids, is therefore particularly interesting in biomedical applications for which multiple different functional moieties on a single discrete macromolecule are needed.
Abstract The synthesis of sequence-defined, discrete star-shaped macromolecules is a major challenge due to the lack of straightforward and versatile approaches. Here, a robust strategy is proposed that allows not only the preparation of sequence-defined mikto-arm star-shaped macromolecules but also the synthesis of a series of unprecedented discrete, multifunctional complex architectures with molar masses above 11 kDa. The iterative approach reported makes use of readily available building blocks and results in asymmetrically branched macromolecules with high purity and yields, which is showcased with monodisperse mikto-arm three-, four-, and five-arm star-shaped structures that were all characterized via LC–MS, MALDI-ToF, and NMR. This effective strategy drastically improves upon synthetic abilities of polymer chemists by enabling simultaneously sequence definition, precision insertion of branching points, as well as the orthogonal end-group functionalization of complex polymeric architectures. The presented approach, which can be translated to different platforms such as peptides and peptoids, is therefore particularly interesting in biomedical applications for which multiple different functional moieties on a single discrete macromolecule are needed.
Sequencing of Uniform Multifunctional Oligoesters via Random Chain Cleavages
M. Soete, F.E. Du Prez
Angew. Chem., 2022
M. Soete, F.E. Du Prez
Angew. Chem., 2022
 Abstract Sequence-defined polymers have been the object of many fascinating studies that focus on their implementation in both material and life science applications. In parallel, iterative synthetic methodologies have become more efficient, whereas the structure elucidation of these molecules is generally dependent on MS/MS analysis. Here, we report an alternative, simple strategy for the determination of the monomer order of uniform oligo(thioether ester)s. This approach, which relies on random cleavages of ester units within the macromolecular backbone via a basic treatment, enables the swift characterization of these macromolecules without the need for MS/MS. Consequently, this method can be used for decoding any information stored within the primary structure of oligoesters by means of ESI- or LC–MS. Finally, we speculate that a range of structurally diverse backbones could be susceptible towards this approach, which could promptly expand the library of chemically sequenceable macromolecules.
Abstract Sequence-defined polymers have been the object of many fascinating studies that focus on their implementation in both material and life science applications. In parallel, iterative synthetic methodologies have become more efficient, whereas the structure elucidation of these molecules is generally dependent on MS/MS analysis. Here, we report an alternative, simple strategy for the determination of the monomer order of uniform oligo(thioether ester)s. This approach, which relies on random cleavages of ester units within the macromolecular backbone via a basic treatment, enables the swift characterization of these macromolecules without the need for MS/MS. Consequently, this method can be used for decoding any information stored within the primary structure of oligoesters by means of ESI- or LC–MS. Finally, we speculate that a range of structurally diverse backbones could be susceptible towards this approach, which could promptly expand the library of chemically sequenceable macromolecules.
Rewritable Macromolecular Data Storage with Automated Read-out
M. Soete, K. De Bruycker, F.E.Du Prez
Angew. Chem., 2022
M. Soete, K. De Bruycker, F.E.Du Prez
Angew. Chem., 2022
 Abstract Rewriting data stored on synthetic macromolecules is an interesting feature, even though it is considered as being quite challenging within the area of digital macromolecules. In this context, we initially studied a strategy for modifying the position tag of sequence-encoded macromolecules in a reversible manner. The efficiency of this method, which relies on the orthogonal cleavage of a thioester moiety via aminolysis, was demonstrated by modifying parts of an exemplary sentence. Simultaneously, a novel algorithm was developed to ease the read-out of macromolecular information by means of MS/MS techniques. This program, Oligoreader, can identify potential information-containing macromolecules from a series of MS 1 spectra, analyze the corresponding MS 2 spectra, and finally decode the data. Consequently, the algorithm simplifies the entire read-out process by avoiding any interference from the operator, which increases the potential for blind sequencing of uniform macromolecules.
Abstract Rewriting data stored on synthetic macromolecules is an interesting feature, even though it is considered as being quite challenging within the area of digital macromolecules. In this context, we initially studied a strategy for modifying the position tag of sequence-encoded macromolecules in a reversible manner. The efficiency of this method, which relies on the orthogonal cleavage of a thioester moiety via aminolysis, was demonstrated by modifying parts of an exemplary sentence. Simultaneously, a novel algorithm was developed to ease the read-out of macromolecular information by means of MS/MS techniques. This program, Oligoreader, can identify potential information-containing macromolecules from a series of MS 1 spectra, analyze the corresponding MS 2 spectra, and finally decode the data. Consequently, the algorithm simplifies the entire read-out process by avoiding any interference from the operator, which increases the potential for blind sequencing of uniform macromolecules.
Combining vinylogous urethane and β-amino ester chemistry for dynamic material design
J.O. Holloway, C. Taplan, F.E. Du Prez
Polym. Chem., 2022
J.O. Holloway, C. Taplan, F.E. Du Prez
Polym. Chem., 2022
 Abstract This study combines vinylogous urethane (VU) and β-amino ester chemistry for the synthesis of covalent adaptable networks (CANs). The resulting CANs are synthesised using a range of diacetoacetates and commercially available diacrylates along with tris(2-aminoethyl)amine, which functions as both amine and crosslinker. The CANs are extensively analysed to determine both their thermal and rheological properties. Several re-processable elastomeric materials are obtained, thanks to the use of polypropylene glycol-containing diacetoacetates of varying molecular weights and are analysed in more detail and compared with VU and amino-ester reference materials. Frequency sweep measurements show no noticeable drop in storage modulus of these CANs between 100–180 °C, indicating a maintained crosslink density. The elastomeric CANs are recycled multiple times, exhibiting no clear loss of dynamic behaviour or any obvious side-reactions.
Abstract This study combines vinylogous urethane (VU) and β-amino ester chemistry for the synthesis of covalent adaptable networks (CANs). The resulting CANs are synthesised using a range of diacetoacetates and commercially available diacrylates along with tris(2-aminoethyl)amine, which functions as both amine and crosslinker. The CANs are extensively analysed to determine both their thermal and rheological properties. Several re-processable elastomeric materials are obtained, thanks to the use of polypropylene glycol-containing diacetoacetates of varying molecular weights and are analysed in more detail and compared with VU and amino-ester reference materials. Frequency sweep measurements show no noticeable drop in storage modulus of these CANs between 100–180 °C, indicating a maintained crosslink density. The elastomeric CANs are recycled multiple times, exhibiting no clear loss of dynamic behaviour or any obvious side-reactions.
Introduction to molecularly defined polymers: synthesis and function
J.A. Johnson, F.E. Du Prez, E. Elacqua
Polym. Chem., 2022
J.A. Johnson, F.E. Du Prez, E. Elacqua
Polym. Chem., 2022
 Abstract Scientists have long been inspired to synthesize macromolecules with the precision and structural complexity of nature. Whereas nature integrates covalent and non-covalent interactions, programs handedness, and controls atomic connectivity with high fidelity, realizing fully synthetic systems that rival these structures remains a daunting challenge. On the other hand, new strategies that synthesize polymers with nature-inspired molecularly-defined structures promise to achieve the next generation of functional materials with properties that could not be achieved with non-discrete polymeric structures. Polymer chemists are looking world-wide for synthetic strategies that feature an ability to achieve: (i) precise atom connectivity (e.g., primary sequence control and/or definition), and/or (ii) precise secondary or tertiary structures (e.g., helices) and (iii) three-dimensional functional group presentation (i.e., stereochemistry, or more commonly “tacticity” in polymer science). In this context, this themed collection, with 2 mini-reviews and 22 research papers, presents a collection of original contributions covering the latest developments in the synthesis and application of oligomers and polymers with controlled, defined, and/or precise molecular scale structures.
Abstract Scientists have long been inspired to synthesize macromolecules with the precision and structural complexity of nature. Whereas nature integrates covalent and non-covalent interactions, programs handedness, and controls atomic connectivity with high fidelity, realizing fully synthetic systems that rival these structures remains a daunting challenge. On the other hand, new strategies that synthesize polymers with nature-inspired molecularly-defined structures promise to achieve the next generation of functional materials with properties that could not be achieved with non-discrete polymeric structures. Polymer chemists are looking world-wide for synthetic strategies that feature an ability to achieve: (i) precise atom connectivity (e.g., primary sequence control and/or definition), and/or (ii) precise secondary or tertiary structures (e.g., helices) and (iii) three-dimensional functional group presentation (i.e., stereochemistry, or more commonly “tacticity” in polymer science). In this context, this themed collection, with 2 mini-reviews and 22 research papers, presents a collection of original contributions covering the latest developments in the synthesis and application of oligomers and polymers with controlled, defined, and/or precise molecular scale structures.
Introduction to the themed collection on synthetic methodologies for complex macromolecular structures in honour of Prof. Yusuf Yagci's 70th birthday
H. Mutlu, F.E. Du Prez, C.R. Becer
Polym. Chem., 2022
H. Mutlu, F.E. Du Prez, C.R. Becer
Polym. Chem., 2022
 Abstract In this themed collection of Polymer Chemistry, we celebrate Professor Yusuf Yagci's 70th birthday, his impact on the development of synthetic methodologies for complex macromolecular architectures and his commitment to diversity, education, and the polymer chemistry community. For more than 4 decades, Yusuf has had a tremendous impact on the advancement of many fields, including polymer synthesis, macromolecular engineering and applied materials science. By following his own quote, “Do not follow others, create your own strategy”, Yusuf has published over 700 papers in peer-reviewed journals with a total of over 35 000 citations, and is a co-inventor on 10 patents.
Importantly, Yusuf has always stated that he is one of the luckiest academics because he has always had brilliant students and scientists in his team. Hence, his scientific creativity, as well as his contribution to the field of polymer science, has not only inspired many Turkish young scientists to follow his steps and enabled this field to become prosperous in Turkey, but has also had a profound effect on conducting creative global research.
Abstract In this themed collection of Polymer Chemistry, we celebrate Professor Yusuf Yagci's 70th birthday, his impact on the development of synthetic methodologies for complex macromolecular architectures and his commitment to diversity, education, and the polymer chemistry community. For more than 4 decades, Yusuf has had a tremendous impact on the advancement of many fields, including polymer synthesis, macromolecular engineering and applied materials science. By following his own quote, “Do not follow others, create your own strategy”, Yusuf has published over 700 papers in peer-reviewed journals with a total of over 35 000 citations, and is a co-inventor on 10 patents.
Importantly, Yusuf has always stated that he is one of the luckiest academics because he has always had brilliant students and scientists in his team. Hence, his scientific creativity, as well as his contribution to the field of polymer science, has not only inspired many Turkish young scientists to follow his steps and enabled this field to become prosperous in Turkey, but has also had a profound effect on conducting creative global research.
Sustainable design of vanillin-based vitrimers using vinylogous urethane chemistry
S. Engelen, A.A. Wróblewska, K. De Bruycker, R.Aksakal, V. Ladmiral, S. Caillol, F.E. Du Prez
Polym. Chem., 2022
S. Engelen, A.A. Wróblewska, K. De Bruycker, R.Aksakal, V. Ladmiral, S. Caillol, F.E. Du Prez
Polym. Chem., 2022
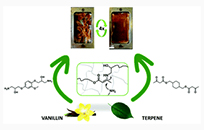 Abstract Research on bio-based covalent adaptable networks is popular nowadays in the search for an optimal implementation of thermoset materials and composites in a circular context. Herein, a vanillin derivative is integrated into vitrimers with promising material properties in which the vinylogous urethane associative chemistry has been used as a dynamic covalent chemistry platform. The vanillin derivative, 2-methoxyhydroquinone, is epoxidised and aminated by aqueous ammonia, with the formation of a bi-functional aromatic β-hydroxy-amine. The straightforward synthesis protocol is high yielding and up-scalable, without the need for any chromatographic purification step. The presented rigid, catalyst-free vitrimers have a high renewable carbon content (up to 86%), glass transition temperatures up to 80 °C and show very fast reprocessing and consequently a swift recyclability with relaxation times in the range of seconds by virtue of the applied β-hydroxy-amine functionality. This research thus provides a sustainable approach for the synthesis of vanillin-based vitrimers and fits in with growing interest for the design of recyclable crosslinked polymer materials.
Abstract Research on bio-based covalent adaptable networks is popular nowadays in the search for an optimal implementation of thermoset materials and composites in a circular context. Herein, a vanillin derivative is integrated into vitrimers with promising material properties in which the vinylogous urethane associative chemistry has been used as a dynamic covalent chemistry platform. The vanillin derivative, 2-methoxyhydroquinone, is epoxidised and aminated by aqueous ammonia, with the formation of a bi-functional aromatic β-hydroxy-amine. The straightforward synthesis protocol is high yielding and up-scalable, without the need for any chromatographic purification step. The presented rigid, catalyst-free vitrimers have a high renewable carbon content (up to 86%), glass transition temperatures up to 80 °C and show very fast reprocessing and consequently a swift recyclability with relaxation times in the range of seconds by virtue of the applied β-hydroxy-amine functionality. This research thus provides a sustainable approach for the synthesis of vanillin-based vitrimers and fits in with growing interest for the design of recyclable crosslinked polymer materials.
Internal catalysis on the opposite side of the fence in non-isocyanate polyurethane covalent adaptable networks
A. Hernández, H.A.Houck, F. Elizalde, M. Guerre, H. Sardon, F.E.Du Prez
Eur. Polym. J., 2022
A. Hernández, H.A.Houck, F. Elizalde, M. Guerre, H. Sardon, F.E.Du Prez
Eur. Polym. J., 2022
 Abstract Within the context of covalent adaptable networks (CANs), we developed in this study a novel methodology to provide non-isocyanate polyurethane-based CANs with embedded tertiary amines that serve as internal catalytic moieties for the dynamic bond exchange processes. For the CAN design, we made use of multifunctional N-substituted 8-membered cyclic carbonates that are ring-opened by macromolecular amines. Several model reactions were conducted to investigate transcarbamoylation bond exchange reactions at elevated temperatures and assess the influence of catalytic moieties within the urethane structure. This led us to design a non-isocyanate polyurethane CAN wherein the position of the internal catalyst was changed with respect to previous reported polyhydroxyurethane CANs, while maintaining close proximity to the dynamic carbamate linkages. It is shown that this positioning change of the tertiary amines still resulted in an internal catalytic effect on the dynamic exchange reactions, hence providing a better understanding of the role of tertiary amines as internal catalysts during the reprocessing of polyurethane networks.
Abstract Within the context of covalent adaptable networks (CANs), we developed in this study a novel methodology to provide non-isocyanate polyurethane-based CANs with embedded tertiary amines that serve as internal catalytic moieties for the dynamic bond exchange processes. For the CAN design, we made use of multifunctional N-substituted 8-membered cyclic carbonates that are ring-opened by macromolecular amines. Several model reactions were conducted to investigate transcarbamoylation bond exchange reactions at elevated temperatures and assess the influence of catalytic moieties within the urethane structure. This led us to design a non-isocyanate polyurethane CAN wherein the position of the internal catalyst was changed with respect to previous reported polyhydroxyurethane CANs, while maintaining close proximity to the dynamic carbamate linkages. It is shown that this positioning change of the tertiary amines still resulted in an internal catalytic effect on the dynamic exchange reactions, hence providing a better understanding of the role of tertiary amines as internal catalysts during the reprocessing of polyurethane networks.
Photo-Crosslinking and Reductive Decrosslinking of
Polymethacrylate-Based Copolymers Containing
1,2-Dithiolane Rings
S. Maes, V. Scholiers, F.E. Du Prez
Macromol. Chem. Phys., 2022
S. Maes, V. Scholiers, F.E. Du Prez
Macromol. Chem. Phys., 2022
 Abstract This study first describes a straightforward approach for the preparation of polymethacrylate-based networks containing variable amounts of lipoates. For this purpose, butyl methacrylate and a 1,2-dithiolane derivative of hydroxymethylmethacrylate are copolymerized in different ratios by free-radical polymerization with the formation of linear network precursors. After optimizing the copolymerization conditions, the ultraviolet (UV)-induced crosslinking behavior is studied. The latter is possible because of the presence of 1,2-dithiolane moieties, which can be photolytically cleaved and recombined upon UV-irradiation. The obtained networks are analyzed in terms of swelling degree, gel fraction, and rheological behavior. Then, network decrosslinking is achieved by immersing the networks in a reducing environment. Finally, successful preliminary coating experiments are conducted, demonstrating the potential applicability of the studied materials.
Abstract This study first describes a straightforward approach for the preparation of polymethacrylate-based networks containing variable amounts of lipoates. For this purpose, butyl methacrylate and a 1,2-dithiolane derivative of hydroxymethylmethacrylate are copolymerized in different ratios by free-radical polymerization with the formation of linear network precursors. After optimizing the copolymerization conditions, the ultraviolet (UV)-induced crosslinking behavior is studied. The latter is possible because of the presence of 1,2-dithiolane moieties, which can be photolytically cleaved and recombined upon UV-irradiation. The obtained networks are analyzed in terms of swelling degree, gel fraction, and rheological behavior. Then, network decrosslinking is achieved by immersing the networks in a reducing environment. Finally, successful preliminary coating experiments are conducted, demonstrating the potential applicability of the studied materials.
Suppressing Creep and Promoting Fast Reprocessing of Vitrimers with Reversibly Trapped Amines
F. Van Lijsebetten, K. De Bruycker, Y. Spiesschaert, J. Winne, F.E. Du Prez
Angew. Chem. Int. Ed., 2022
F. Van Lijsebetten, K. De Bruycker, Y. Spiesschaert, J. Winne, F.E. Du Prez
Angew. Chem. Int. Ed., 2022
 Abstract We report a straightforward chemical strategy to tackle current challenges of irreversible deformation in low Tg vitrimers at operating temperature. In particular, vinylogous urethane (VU) vitrimers were prepared where reactive free amines, necessary for material flow, were temporarily shielded inside the network backbone, by adding a small amount of dibasic ester to the curing mixture. The amines could be released as reactive chain ends from the resulting dicarboxamide bonds via thermally reversible cyclisation to an imide moiety. Indeed, (re)generation of the required nucleophilic amines as network defects ensured reprocessing and rapid material flow at higher temperature, where exchange dynamics are (re)activated. As a result, VU vitrimers were obtained with limited creep at service temperature, yet with good reprocessability at elevated temperatures. Thus, by exerting strong control on the molecular level over the availability of exchangeable functional groups, a remarkable improvement of VU properties was obtained.
Abstract We report a straightforward chemical strategy to tackle current challenges of irreversible deformation in low Tg vitrimers at operating temperature. In particular, vinylogous urethane (VU) vitrimers were prepared where reactive free amines, necessary for material flow, were temporarily shielded inside the network backbone, by adding a small amount of dibasic ester to the curing mixture. The amines could be released as reactive chain ends from the resulting dicarboxamide bonds via thermally reversible cyclisation to an imide moiety. Indeed, (re)generation of the required nucleophilic amines as network defects ensured reprocessing and rapid material flow at higher temperature, where exchange dynamics are (re)activated. As a result, VU vitrimers were obtained with limited creep at service temperature, yet with good reprocessability at elevated temperatures. Thus, by exerting strong control on the molecular level over the availability of exchangeable functional groups, a remarkable improvement of VU properties was obtained.
Reprocessing of Covalent Adaptable Polyamide Networks through Internal Catalysis and Ring-Size Effects
F. Van Lijsebetten, Y. Spiesschaert, J.M. Winne, F.E. Du Prez
J. Am. Chem. Soc. 2021
F. Van Lijsebetten, Y. Spiesschaert, J.M. Winne, F.E. Du Prez
J. Am. Chem. Soc. 2021
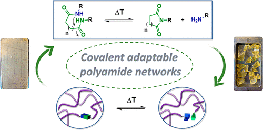 Abstract Here, we report the introduction of internally catalyzed amide bonds to obtain covalent adaptable polyamide networks that rely on the dissociation equilibrium between dicarboxamides and imides. While amide bonds are usually considered to be robust and thermally stable, the present study shows that their dynamic character can be activated by a smart choice of available building blocks without the addition of any external catalyst or other additives. Hence, a range of polyamide-based dynamic networks with variable mechanical and viscoelastic properties have been obtained in a systematic study, using a straightforward curing process of dibasic ester and amine compounds. Since the dissociation process involves a cyclic imide formation, the correlation between ring size and the thermomechanical viscosity profile was studied for five- to seven-membered ring intermediates, depending on the chosen dibasic ester monomer. This resulted in a marked temperature response with activation energies in the range of 116–197 kJ mol–1, yielding a sharp transition between elastic and viscous behavior. Moreover, the ease and versatility of this chemistry platform were demonstrated by selecting a variety of amines, resulting in densely cross-linked dynamic networks with Tg values ranging from −20 to 110 °C.
Abstract Here, we report the introduction of internally catalyzed amide bonds to obtain covalent adaptable polyamide networks that rely on the dissociation equilibrium between dicarboxamides and imides. While amide bonds are usually considered to be robust and thermally stable, the present study shows that their dynamic character can be activated by a smart choice of available building blocks without the addition of any external catalyst or other additives. Hence, a range of polyamide-based dynamic networks with variable mechanical and viscoelastic properties have been obtained in a systematic study, using a straightforward curing process of dibasic ester and amine compounds. Since the dissociation process involves a cyclic imide formation, the correlation between ring size and the thermomechanical viscosity profile was studied for five- to seven-membered ring intermediates, depending on the chosen dibasic ester monomer. This resulted in a marked temperature response with activation energies in the range of 116–197 kJ mol–1, yielding a sharp transition between elastic and viscous behavior. Moreover, the ease and versatility of this chemistry platform were demonstrated by selecting a variety of amines, resulting in densely cross-linked dynamic networks with Tg values ranging from −20 to 110 °C.
Polyaddition synthesis using alkyne esters for the design of vinylogous urethane vitrimers
Y. Spiesschaert, J. Danneels, N. Van Herck, M. Guerre, G. Acke, J.M. Winne, F.E. Du Prez
Macromolecules 2021, 54, 17, 7931–7942
Y. Spiesschaert, J. Danneels, N. Van Herck, M. Guerre, G. Acke, J.M. Winne, F.E. Du Prez
Macromolecules 2021, 54, 17, 7931–7942
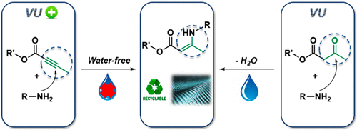 Abstract Vitrimers are a subclass of covalent adaptable networks which introduce reshapeability and recyclability in thermoset materials while maintaining a high degree of chemical resistance and dimensional stability. Vitrimer materials based on vinylogous urethane (VU) chemistry have drawn a lot of attention in this area. Classically, these are obtained by the polycondensation polymerization of acetoacetate and amine monomers. Unfortunately, this also releases water, often leading to porosity defects in the initially obtained non-reprocessed cross-linked materials. Here, we demonstrate that alkyne esters (AE) can be used as alternative building blocks for VU vitrimers by a polyaddition polymerization with amines, leading to water-free formulations and straightforward access to defect-free cured VU vitrimer materials. The bond formation and dynamic bond exchange was also studied by small molecule reactions, further rationalized by a computational (DFT) approach. The resulting water-free VU vitrimers display similar material properties compared to vitrimers based on acetoacetates, although also some differences are seen, which can be related to a minor amide-bond forming side reaction.
Abstract Vitrimers are a subclass of covalent adaptable networks which introduce reshapeability and recyclability in thermoset materials while maintaining a high degree of chemical resistance and dimensional stability. Vitrimer materials based on vinylogous urethane (VU) chemistry have drawn a lot of attention in this area. Classically, these are obtained by the polycondensation polymerization of acetoacetate and amine monomers. Unfortunately, this also releases water, often leading to porosity defects in the initially obtained non-reprocessed cross-linked materials. Here, we demonstrate that alkyne esters (AE) can be used as alternative building blocks for VU vitrimers by a polyaddition polymerization with amines, leading to water-free formulations and straightforward access to defect-free cured VU vitrimer materials. The bond formation and dynamic bond exchange was also studied by small molecule reactions, further rationalized by a computational (DFT) approach. The resulting water-free VU vitrimers display similar material properties compared to vitrimers based on acetoacetates, although also some differences are seen, which can be related to a minor amide-bond forming side reaction.
Sequence-defined oligoampholytes using hydrolytically stable vinyl sulfonamides: design and UCST behaviour
C. Mertens, R. Aksakal, N. Badi, F.E. Du Prez
Polym. Chem., 2021, 12, 4193-4204
C. Mertens, R. Aksakal, N. Badi, F.E. Du Prez
Polym. Chem., 2021, 12, 4193-4204
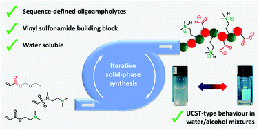 Abstract Polyampholytes, widely investigated for their distinct properties, are typically prepared via conventional polymerisation techniques. This results in an ensemble of polymer chains with variation in molecular parameters such as length, ratio of charged groups and monomer order, which could influence their behaviour. Here, uniform oligoampholytes with precisely positioned negatively charged carboxylate and positively charged ammonium side-chains were synthesised using an iterative solid-phase synthesis strategy based on thiolactone chemistry. The amine side-chains were initially introduced via an acrylate, resulting in an amino-functionalised β-thioester that was shown to be susceptible to transesterification and hydrolysis, even under ambient conditions. While increasing the spacer length between the β-thioester and amine functionality could slow down this undesired side-reaction, it could not be completely suppressed. On the other hand, a tertiary amine-bearing vinyl sulfonamide proved to be a viable, hydrolytically stable alternative to introduce this moiety. The resulting uniform oligoampholytes are soluble in water and show UCST-type thermoresponsive behaviour in 85 vol% isopropanol/water mixtures.
Abstract Polyampholytes, widely investigated for their distinct properties, are typically prepared via conventional polymerisation techniques. This results in an ensemble of polymer chains with variation in molecular parameters such as length, ratio of charged groups and monomer order, which could influence their behaviour. Here, uniform oligoampholytes with precisely positioned negatively charged carboxylate and positively charged ammonium side-chains were synthesised using an iterative solid-phase synthesis strategy based on thiolactone chemistry. The amine side-chains were initially introduced via an acrylate, resulting in an amino-functionalised β-thioester that was shown to be susceptible to transesterification and hydrolysis, even under ambient conditions. While increasing the spacer length between the β-thioester and amine functionality could slow down this undesired side-reaction, it could not be completely suppressed. On the other hand, a tertiary amine-bearing vinyl sulfonamide proved to be a viable, hydrolytically stable alternative to introduce this moiety. The resulting uniform oligoampholytes are soluble in water and show UCST-type thermoresponsive behaviour in 85 vol% isopropanol/water mixtures.
Using nickel to fold discrete synthetic macromolecules into single-chain nanoparticles
M.A. Reith, S. Kardas, C. Mertens, M. Fossépré, M. Surin, J. Steinkoenig, F.E. Du Prez
Polym. Chem., 2021, 12, 4924-4933
M.A. Reith, S. Kardas, C. Mertens, M. Fossépré, M. Surin, J. Steinkoenig, F.E. Du Prez
Polym. Chem., 2021, 12, 4924-4933
 Abstract Macromolecules found in Nature display a precise control over the primary as well as higher ordered architectures. To mimic the folding found in Nature, we herein demonstrate the design and characterization of single-chain nanoparticles that are formed by the folding of sequence-defined macromolecules with metal ions. The study showcases the influence of the loop size of such precision macromolecules on their relative hydrodynamic radius. The sequence-defined structures are fabricated using thiolactone chemistry, where two picolyl moieties are installed forming a valuable ligand system for subsequent metal complexation. Next, metal ions such as Ni(II) and Cu(II) ions are introduced to fold the unimers into sequence-defined single-chain nanoparticles (SD-SCNPs). After proving the successful complexation using a trimer, a systematic study is conducted altering the distance between the respective ligands by incorporating variable numbers of non-functionalized spacer units. Finally, the loop size formation of the SD-SCNPs is evidenced by DOSY measurements. The result indicates that the positioning of the ligands plays a crucial role on the compaction process and, more specifically, on the final size of the SD-SCNP. In addition, molecular dynamics (MD) simulations show the effects of the sequence and Ni(II) complexation on the structure and compaction of the SD-SCNPs, and highlight the differences of the nanoparticles’ shape when varying the number of spacer units.
Abstract Macromolecules found in Nature display a precise control over the primary as well as higher ordered architectures. To mimic the folding found in Nature, we herein demonstrate the design and characterization of single-chain nanoparticles that are formed by the folding of sequence-defined macromolecules with metal ions. The study showcases the influence of the loop size of such precision macromolecules on their relative hydrodynamic radius. The sequence-defined structures are fabricated using thiolactone chemistry, where two picolyl moieties are installed forming a valuable ligand system for subsequent metal complexation. Next, metal ions such as Ni(II) and Cu(II) ions are introduced to fold the unimers into sequence-defined single-chain nanoparticles (SD-SCNPs). After proving the successful complexation using a trimer, a systematic study is conducted altering the distance between the respective ligands by incorporating variable numbers of non-functionalized spacer units. Finally, the loop size formation of the SD-SCNPs is evidenced by DOSY measurements. The result indicates that the positioning of the ligands plays a crucial role on the compaction process and, more specifically, on the final size of the SD-SCNP. In addition, molecular dynamics (MD) simulations show the effects of the sequence and Ni(II) complexation on the structure and compaction of the SD-SCNPs, and highlight the differences of the nanoparticles’ shape when varying the number of spacer units.
Covalent Adaptable Networks Using β-Amino Esters as Thermally Reversible Building Blocks
C. Taplan, M. Guerre, F.E. Du Prez
J. Am. Chem. Soc., 2021, 143, 24, 9140–9150
C. Taplan, M. Guerre, F.E. Du Prez
J. Am. Chem. Soc., 2021, 143, 24, 9140–9150
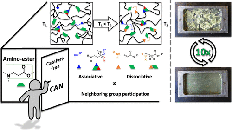 Abstract In this study, β-amino esters, prepared by the aza-Michael addition of an amine to an acrylate moiety, are investigated as building blocks for the formation of dynamic covalent networks. While such amino esters are usually considered as thermally nondynamic adducts, the kinetic model studies presented here show that dynamic covalent exchange occurs via both dynamic aza-Michael reaction and catalyst-free transesterification. This knowledge is transferred to create β-amino ester-based covalent adaptable networks (CANs) with coexisting dissociative and associative covalent dynamic exchange reactions. The ease, robustness, and versatility of this chemistry are demonstrated by using a variety of readily available multifunctional acrylates and amines. The presented CANs are reprocessed via either a dynamic aza-Michael reaction or a catalyst-free transesterification in the presence of hydroxyl moieties. This results in reprocessable, densely cross-linked materials with a glass transition temperature (Tg) ranging from −60 to 90 °C. Moreover, even for the low Tg materials, a high creep resistance was demonstrated at elevated temperatures up to 80 °C. When additional β-hydroxyl group-containing building blocks are applied during the network design, an enhanced neighboring group participation effect allows reprocessing of materials up to 10 times at 150 °C within 30 min while maintaining their material properties.
Abstract In this study, β-amino esters, prepared by the aza-Michael addition of an amine to an acrylate moiety, are investigated as building blocks for the formation of dynamic covalent networks. While such amino esters are usually considered as thermally nondynamic adducts, the kinetic model studies presented here show that dynamic covalent exchange occurs via both dynamic aza-Michael reaction and catalyst-free transesterification. This knowledge is transferred to create β-amino ester-based covalent adaptable networks (CANs) with coexisting dissociative and associative covalent dynamic exchange reactions. The ease, robustness, and versatility of this chemistry are demonstrated by using a variety of readily available multifunctional acrylates and amines. The presented CANs are reprocessed via either a dynamic aza-Michael reaction or a catalyst-free transesterification in the presence of hydroxyl moieties. This results in reprocessable, densely cross-linked materials with a glass transition temperature (Tg) ranging from −60 to 90 °C. Moreover, even for the low Tg materials, a high creep resistance was demonstrated at elevated temperatures up to 80 °C. When additional β-hydroxyl group-containing building blocks are applied during the network design, an enhanced neighboring group participation effect allows reprocessing of materials up to 10 times at 150 °C within 30 min while maintaining their material properties.
Biobased acrylic pressure-sensitive adhesives
M.A. Droesbeke, R. Aksakal, A. Simula, J.M. Asua, F.E. Du Prez
Prog. Polym. Sci., 2021, 17, 101396
M.A. Droesbeke, R. Aksakal, A. Simula, J.M. Asua, F.E. Du Prez
Prog. Polym. Sci., 2021, 17, 101396
 Abstract The ever-increasing regulations within the adhesives industry and the continuous demand for more sustainability, have led to the development of environmentally more suitable alternatives to petroleum-based pressure-sensitive adhesives (PSAs). This review provides an overview of the current strategies and methods to develop sustainable, acrylic PSAs derived from renewable resources, such as vegetable oil, lignin, carbohydrates and terpenes. Herein, we emphasize some of the key concepts in the literature within the past decades, which paved the way to the current focus of improving the adhesive properties by employing the full array of natural building blocks. In this context, some recent strategies that give tailor-made adhesive properties to PSAs are also discussed, in view of the desired application. With this critical insight on the large set of research efforts in the context of biosourced acrylic PSAs, we aim to provide a guide for new researchers in this highly emerging field.
Abstract The ever-increasing regulations within the adhesives industry and the continuous demand for more sustainability, have led to the development of environmentally more suitable alternatives to petroleum-based pressure-sensitive adhesives (PSAs). This review provides an overview of the current strategies and methods to develop sustainable, acrylic PSAs derived from renewable resources, such as vegetable oil, lignin, carbohydrates and terpenes. Herein, we emphasize some of the key concepts in the literature within the past decades, which paved the way to the current focus of improving the adhesive properties by employing the full array of natural building blocks. In this context, some recent strategies that give tailor-made adhesive properties to PSAs are also discussed, in view of the desired application. With this critical insight on the large set of research efforts in the context of biosourced acrylic PSAs, we aim to provide a guide for new researchers in this highly emerging field.
Assembling Lipoic Acid and Nanoclay into Nacre-Mimetic Nanocomposites
J. Huang, A.A. Wróblewska, J. Steinkoenig, S. Maes, F.E. Du Prez
Macromolecules 2021, 54, 10, 4658–4668
J. Huang, A.A. Wróblewska, J. Steinkoenig, S. Maes, F.E. Du Prez
Macromolecules 2021, 54, 10, 4658–4668
 Abstract A facile strategy to obtain nacre-mimetic nanocomposite materials is presented. The proposed economically feasible and environmentally friendly process consists in fusing dynamic poly(lipoic acid) and montmorillonite (MTM) via a water evaporation method. Lipoic acid (LA), a naturally occurring molecule, can readily undergo thermal-, UV-, or (water) evaporation-induced dynamic ring-opening polymerization. This monomer has been equipped with various organic and inorganic counter cations, altering the physical properties of both the obtained poly(lipoate)s and the resulting nacre-like nanocomposites. The mechanical performance of the nanocomposites can be accordingly tuned from rigid and brittle to soft and ductile. Importantly, the composites containing triethanolammonium cations show enhanced ductility with an elongation at break up to around 16% when compared to other reported nacre-mimetic nanocomposites with similar MTM content (elongation at break usually below 10%). Moreover, the influence of MTM content on the mechanical properties of the nanocomposites is elucidated. Furthermore, the dynamic nature of the poly(lipoate)s and supramolecular architecture within the composites enables water-assisted self-healing of superficial scratches, as evaluated by optical microscopy, and closed-loop recycling for materials with up to 70 wt % MTM.
Abstract A facile strategy to obtain nacre-mimetic nanocomposite materials is presented. The proposed economically feasible and environmentally friendly process consists in fusing dynamic poly(lipoic acid) and montmorillonite (MTM) via a water evaporation method. Lipoic acid (LA), a naturally occurring molecule, can readily undergo thermal-, UV-, or (water) evaporation-induced dynamic ring-opening polymerization. This monomer has been equipped with various organic and inorganic counter cations, altering the physical properties of both the obtained poly(lipoate)s and the resulting nacre-like nanocomposites. The mechanical performance of the nanocomposites can be accordingly tuned from rigid and brittle to soft and ductile. Importantly, the composites containing triethanolammonium cations show enhanced ductility with an elongation at break up to around 16% when compared to other reported nacre-mimetic nanocomposites with similar MTM content (elongation at break usually below 10%). Moreover, the influence of MTM content on the mechanical properties of the nanocomposites is elucidated. Furthermore, the dynamic nature of the poly(lipoate)s and supramolecular architecture within the composites enables water-assisted self-healing of superficial scratches, as evaluated by optical microscopy, and closed-loop recycling for materials with up to 70 wt % MTM.
Sequence-Encoded Macromolecules with Increased Data Storage Capacity through a Thiol-Epoxy Reaction
M. Soete, C. Mertens, R. Aksakal, N. Badi, F.E. Du Prez
ACS Macro Lett. 2021, 10, 5, 616–622
M. Soete, C. Mertens, R. Aksakal, N. Badi, F.E. Du Prez
ACS Macro Lett. 2021, 10, 5, 616–622
 Abstract Sequence-encoded oligo(thioether urethane)s with two different coding monomers per backbone unit were prepared via a solid phase, two-step iterative protocol based on thiolactone chemistry. The first step of the synthetic cycle consists of the thiolactone ring opening with a primary amine, whereby the in situ released thiol is immediately reacted with an epoxide. In the second step, the thiolactone group is reinstalled to initiate the next cycle. This strategy allows to introduce two different coding monomers per synthetic cycle, rendering the resulting macromolecules especially attractive in the area of (macro)molecular data storage because of their increased data storage capacity. Subsequently, the efficiency of the herein reported synthesis route and the applicability of the dual-encoded sequence-defined macromolecules as a potential data storage platform have been demonstrated by unraveling the exact monomer order using tandem mass spectrometry techniques.
Abstract Sequence-encoded oligo(thioether urethane)s with two different coding monomers per backbone unit were prepared via a solid phase, two-step iterative protocol based on thiolactone chemistry. The first step of the synthetic cycle consists of the thiolactone ring opening with a primary amine, whereby the in situ released thiol is immediately reacted with an epoxide. In the second step, the thiolactone group is reinstalled to initiate the next cycle. This strategy allows to introduce two different coding monomers per synthetic cycle, rendering the resulting macromolecules especially attractive in the area of (macro)molecular data storage because of their increased data storage capacity. Subsequently, the efficiency of the herein reported synthesis route and the applicability of the dual-encoded sequence-defined macromolecules as a potential data storage platform have been demonstrated by unraveling the exact monomer order using tandem mass spectrometry techniques.
Applications of Discrete Synthetic Macromolecules in Life and Materials Science: Recent and Future Trends
R. Aksakal, C. Mertens, M.Soete, N. Badi, F.E. Du Prez
Adv. Sci. 2021, 2004038
R. Aksakal, C. Mertens, M.Soete, N. Badi, F.E. Du Prez
Adv. Sci. 2021, 2004038
 Abstract In the last decade, the field of sequence‐defined polymers and related ultraprecise, monodisperse synthetic macromolecules has grown exponentially. In the early stage, mainly articles or reviews dedicated to the development of synthetic routes toward their preparation have been published. Nowadays, those synthetic methodologies, combined with the elucidation of the structure–property relationships, allow envisioning many promising applications. Consequently, in the past 3 years, application‐oriented papers based on discrete synthetic macromolecules emerged. Hence, material science applications such as macromolecular data storage and encryption, self‐assembly of discrete structures and foldamers have been the object of many fascinating studies. Moreover, in the area of life sciences, such structures have also been the focus of numerous research studies. Here, it is aimed to highlight these recent applications and to give the reader a critical overview of the future trends in this area of research.
Abstract In the last decade, the field of sequence‐defined polymers and related ultraprecise, monodisperse synthetic macromolecules has grown exponentially. In the early stage, mainly articles or reviews dedicated to the development of synthetic routes toward their preparation have been published. Nowadays, those synthetic methodologies, combined with the elucidation of the structure–property relationships, allow envisioning many promising applications. Consequently, in the past 3 years, application‐oriented papers based on discrete synthetic macromolecules emerged. Hence, material science applications such as macromolecular data storage and encryption, self‐assembly of discrete structures and foldamers have been the object of many fascinating studies. Moreover, in the area of life sciences, such structures have also been the focus of numerous research studies. Here, it is aimed to highlight these recent applications and to give the reader a critical overview of the future trends in this area of research.
Light-fueled dynamic covalent crosslinking of single polymer chains in non-equilibrium states
D. Kodura, H.A. Houck, F.R. Bloesser, A.S. Goldmann, F.E. Du Prez, H. Frisch, C. Barner-Kowollik
Chem. Sci., 2021, Advance Article
D. Kodura, H.A. Houck, F.R. Bloesser, A.S. Goldmann, F.E. Du Prez, H. Frisch, C. Barner-Kowollik
Chem. Sci., 2021, Advance Article
 Abstract While polymer synthesis proceeds predominantly towards the thermodynamic minimum, living systems operate on the reverse principle – consuming fuel to maintain a non-equilibrium state. Herein, we report the controlled formation of 3D macromolecular architectures based on light-fueled covalent non-equilibrium chemistry. In the presence of green light (525 nm) and a bivalent triazolinedione (TAD) crosslinker, naphthalene-containing polymers can be folded into single chain nanoparticles (SCNPs). At ambient temperature, the cycloaddition product of TAD with naphthalene reverts and the SCNP unfolds into its linear parent polymer. The reported SCNP is the first example of a reversible light triggered folding of single polymer chains and can readily be repeated for several cycles. The folded state of the SCNP can either be preserved through a constant supply of light fuel, kinetic trapping or through a chemical modification that makes the folded state thermodynamically favored. Whereas small molecule bivalent TAD/naphthalene cycloaddition products largely degraded after 3 days in solution, even in the presence of fuel, the SCNP entities were found to remain intact, thereby indicating the light-fueled stabilization of the SCNP to be an inherent feature of the confined macromolecular environment.
Abstract While polymer synthesis proceeds predominantly towards the thermodynamic minimum, living systems operate on the reverse principle – consuming fuel to maintain a non-equilibrium state. Herein, we report the controlled formation of 3D macromolecular architectures based on light-fueled covalent non-equilibrium chemistry. In the presence of green light (525 nm) and a bivalent triazolinedione (TAD) crosslinker, naphthalene-containing polymers can be folded into single chain nanoparticles (SCNPs). At ambient temperature, the cycloaddition product of TAD with naphthalene reverts and the SCNP unfolds into its linear parent polymer. The reported SCNP is the first example of a reversible light triggered folding of single polymer chains and can readily be repeated for several cycles. The folded state of the SCNP can either be preserved through a constant supply of light fuel, kinetic trapping or through a chemical modification that makes the folded state thermodynamically favored. Whereas small molecule bivalent TAD/naphthalene cycloaddition products largely degraded after 3 days in solution, even in the presence of fuel, the SCNP entities were found to remain intact, thereby indicating the light-fueled stabilization of the SCNP to be an inherent feature of the confined macromolecular environment.
Substituent effect on the thermophysical properties and thermal dissociation behaviour of 9-substituted anthracene derivatives
J. Brancart, J. Van Damme, F.E. Du Prez, G. Van Assche
Phys. Chem. Chem. Phys., 2021, Advance Article
J. Brancart, J. Van Damme, F.E. Du Prez, G. Van Assche
Phys. Chem. Chem. Phys., 2021, Advance Article
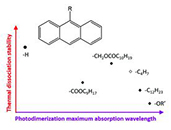 Abstract The chemical structure and location of substituents on anthracene derivatives influence the electron balance of the aromatic system, thus determining the wavelengths at which light is absorbed, which results in the photochemically induced dimerization or monomerization. Here, the thermal dissociation kinetics of 7 photodimers of 9-substituted anthracene derivatives are studied using a combination of spectroscopic and calorimetric techniques in the condensed state and compared to scarce literature data on thermal dissociation of other anthracene derivatives. The length and chemical structure of the substituent chains have a clear impact on the melting temperatures of the anthracene derivatives and corresponding photodimers. The crystallinity of the photodimers and monomers in turn influences the thermal dissociation kinetics. The thermal dissociation behaviour and previously published photochemistry data are related to the electronic effects of the substituents by means of the Hammett parameters. Stronger electron-withdrawing effects result in larger red shifts of the maximum wavelength λmax for the photodimerization of the anthracene derivatives. It is also shown that for the studied substitutions on the 9-position of anthracene, the higher the magnitude of the electronic effect – both electron-donating and electron-withdrawing – the faster the thermal dissociation kinetics and thus the lower the thermal stability.
Abstract The chemical structure and location of substituents on anthracene derivatives influence the electron balance of the aromatic system, thus determining the wavelengths at which light is absorbed, which results in the photochemically induced dimerization or monomerization. Here, the thermal dissociation kinetics of 7 photodimers of 9-substituted anthracene derivatives are studied using a combination of spectroscopic and calorimetric techniques in the condensed state and compared to scarce literature data on thermal dissociation of other anthracene derivatives. The length and chemical structure of the substituent chains have a clear impact on the melting temperatures of the anthracene derivatives and corresponding photodimers. The crystallinity of the photodimers and monomers in turn influences the thermal dissociation kinetics. The thermal dissociation behaviour and previously published photochemistry data are related to the electronic effects of the substituents by means of the Hammett parameters. Stronger electron-withdrawing effects result in larger red shifts of the maximum wavelength λmax for the photodimerization of the anthracene derivatives. It is also shown that for the studied substitutions on the 9-position of anthracene, the higher the magnitude of the electronic effect – both electron-donating and electron-withdrawing – the faster the thermal dissociation kinetics and thus the lower the thermal stability.
Surface Modification of (Non)‐Fluorinated Vitrimers through Dynamic Transamination
C.Taplan, M. Guerre, C.N. Bowman, F.E. Du Prez
Macromol. Rapid Commun., 2020, 2000644
C.Taplan, M. Guerre, C.N. Bowman, F.E. Du Prez
Macromol. Rapid Commun., 2020, 2000644
 Abstract Surface modifications are typically permanent in shape and chemistry. Herein, vinylogous urethane (VU) chemistry is presented as an easily accessible and versatile platform for rapid, facile, and reworkable surface modification. It is demonstrated that both physical and chemical post‐modification of permanent, yet dynamic elastic polymer networks are achieved. Surface patterns with high regularity are created, both via a straightforward replication process using a polydimethylsiloxane stamp (resolution ca. 10–100 µm) as well as using thermally activated nano‐imprint lithography (NIL) to form hole, pillar, or line patterns (ca. 300 nm) in elastic VU‐based vitrimers. The tunable, rapid exchange allows patterning at 130 °C in less than 15 min, resulting in an increased water contact angle and surface‐structure induced light reflection. Moreover, it is also demonstrated that the use of a single dynamic covalent chemistry makes it possible to strongly adhere to fluorinated and non‐fluorinated materials based on incompatible matrices.
Abstract Surface modifications are typically permanent in shape and chemistry. Herein, vinylogous urethane (VU) chemistry is presented as an easily accessible and versatile platform for rapid, facile, and reworkable surface modification. It is demonstrated that both physical and chemical post‐modification of permanent, yet dynamic elastic polymer networks are achieved. Surface patterns with high regularity are created, both via a straightforward replication process using a polydimethylsiloxane stamp (resolution ca. 10–100 µm) as well as using thermally activated nano‐imprint lithography (NIL) to form hole, pillar, or line patterns (ca. 300 nm) in elastic VU‐based vitrimers. The tunable, rapid exchange allows patterning at 130 °C in less than 15 min, resulting in an increased water contact angle and surface‐structure induced light reflection. Moreover, it is also demonstrated that the use of a single dynamic covalent chemistry makes it possible to strongly adhere to fluorinated and non‐fluorinated materials based on incompatible matrices.
Internal catalysis for dynamic covalent chemistry applications and polymer science
F. Van Lijsebetten, J.O. Holloway, J.M. Winne, F.E. Du Prez
Chem. Soc. Rev., 2020
F. Van Lijsebetten, J.O. Holloway, J.M. Winne, F.E. Du Prez
Chem. Soc. Rev., 2020
 Abstract Strong covalent chemical bonds that can also be reversed, cleaved or exchanged are the subject of so-called dynamic covalent chemistry (DCC). Applications range from classical protective groups in organic chemistry and cleavable linkers for solid phase synthesis, to more modern applications in dynamic compound libraries and adaptive materials. Interest in dynamic, reversible or responsive chemistries has risen in particular in the last few decades for the design and synthesis of new DCC-based polymer materials. Implementation of DCC in polymers yields materials with unique combinations of properties and in some cases even unprecedented properties for covalent materials, such as self-healing materials, covalent adaptable networks (CANs) and vitrimers. In particular, the incorporation of DCC in polymer materials aims to find a balance between a swift and triggerable reactivity, combined with a high degree of intrinsic robustness and stability. Applying harsh conditions, highly active catalysts or highly reactive bonding groups, as is done in classical DCC, is often not feasible or desirable, as it can damage the polymer's integrity, leading to loss of function and properties. In this context, so-called internally catalysed DCC platforms have started to receive more interest in this area. This approach relies on the relative proximity and orientation of common functional groups, which can influence a chemical exchange reaction in a subtle but significant way...
Abstract Strong covalent chemical bonds that can also be reversed, cleaved or exchanged are the subject of so-called dynamic covalent chemistry (DCC). Applications range from classical protective groups in organic chemistry and cleavable linkers for solid phase synthesis, to more modern applications in dynamic compound libraries and adaptive materials. Interest in dynamic, reversible or responsive chemistries has risen in particular in the last few decades for the design and synthesis of new DCC-based polymer materials. Implementation of DCC in polymers yields materials with unique combinations of properties and in some cases even unprecedented properties for covalent materials, such as self-healing materials, covalent adaptable networks (CANs) and vitrimers. In particular, the incorporation of DCC in polymer materials aims to find a balance between a swift and triggerable reactivity, combined with a high degree of intrinsic robustness and stability. Applying harsh conditions, highly active catalysts or highly reactive bonding groups, as is done in classical DCC, is often not feasible or desirable, as it can damage the polymer's integrity, leading to loss of function and properties. In this context, so-called internally catalysed DCC platforms have started to receive more interest in this area. This approach relies on the relative proximity and orientation of common functional groups, which can influence a chemical exchange reaction in a subtle but significant way...
Biosourced terpenoids for the development of sustainable acrylic pressure-sensitive adhesives via emulsion polymerisation
M.A. Droesbeke, A. Simula, J.M. Asuab, F.E. Du Prez
Green Chem., 2020
M.A. Droesbeke, A. Simula, J.M. Asuab, F.E. Du Prez
Green Chem., 2020
 Abstract The increasing regulations and restrictions in favour of a biobased and sustainable community could potentially harm the strong economic position of the polymer industry, which still heavily relyies on crude oil. The adhesive industry, in particular, is looking for more renewable alternatives and more environmentally friendly synthesis routes. In this work, (meth)acrylate derivatives of terpenoids, namely tetrahydrogeraniol, citronellol, menthol and isoborneol are introduced in the synthesis of waterborne pressure-sensitive adhesives (PSA) based on acrylic latexes via emulsion polymerisation. This industrially implemented setting enables the preparation of five different formulations with high biobased content with a renewable carbon content ranging from 70 to 100%. The biobased PSAs are found to be comparable in terms of tack, peel strength and shear resistance to a benchmark petroleum-derived commercial product. They show good adhesion properties on steel, glass and polyethylene surfaces. Moreover, the various formulations displayed different mechanical and adhesion properties, which make them attractive for a wide range of applications.
Abstract The increasing regulations and restrictions in favour of a biobased and sustainable community could potentially harm the strong economic position of the polymer industry, which still heavily relyies on crude oil. The adhesive industry, in particular, is looking for more renewable alternatives and more environmentally friendly synthesis routes. In this work, (meth)acrylate derivatives of terpenoids, namely tetrahydrogeraniol, citronellol, menthol and isoborneol are introduced in the synthesis of waterborne pressure-sensitive adhesives (PSA) based on acrylic latexes via emulsion polymerisation. This industrially implemented setting enables the preparation of five different formulations with high biobased content with a renewable carbon content ranging from 70 to 100%. The biobased PSAs are found to be comparable in terms of tack, peel strength and shear resistance to a benchmark petroleum-derived commercial product. They show good adhesion properties on steel, glass and polyethylene surfaces. Moreover, the various formulations displayed different mechanical and adhesion properties, which make them attractive for a wide range of applications.
Analysis of sequence-defined oligomers through Advanced Polymer Chromatography™ – mass spectrometry hyphenation
M-T. Berga, C. Mertens, F.E. Du Prez, T.D. Kühnea, A.Herberga, D.Kuckling
RSC Adv., 2020
M-T. Berga, C. Mertens, F.E. Du Prez, T.D. Kühnea, A.Herberga, D.Kuckling
RSC Adv., 2020
 Abstract In recent years, sequence-defined oligomers have attracted increasing interest in the polymer community and the number of new applications such as macromolecular data storage and encryption is increasing. However, techniques allowing sequence differentiation are still lacking. In this study, the focus is put towards a new strategy allowing structural distinction between sequence-defined oligomers with identical molecular weight and composition, but bearing different sequences. This technique relies on the hyphenation of size exclusion chromatography and mass spectrometry, coupled with ion mobility separation. This approach allows for a quick and easy separation and identification of oligomers with different length and/or sequence.
Abstract In recent years, sequence-defined oligomers have attracted increasing interest in the polymer community and the number of new applications such as macromolecular data storage and encryption is increasing. However, techniques allowing sequence differentiation are still lacking. In this study, the focus is put towards a new strategy allowing structural distinction between sequence-defined oligomers with identical molecular weight and composition, but bearing different sequences. This technique relies on the hyphenation of size exclusion chromatography and mass spectrometry, coupled with ion mobility separation. This approach allows for a quick and easy separation and identification of oligomers with different length and/or sequence.
Influence of the polymer matrix on the viscoelastic behaviour of vitrimers
Y. Spiesschaert, C. Taplan, L. Stricker, M. Guerre, J.M. Winne, F.E. Du Prez
Polym. Chem., 2020
Y. Spiesschaert, C. Taplan, L. Stricker, M. Guerre, J.M. Winne, F.E. Du Prez
Polym. Chem., 2020
.gif) Abstract Vitrimer materials are a rapidly growing field of research, in which still many fundamental aspects of material design remain to be explored. In this study, we report a systematic study about the effect of the choice of the matrix on a dynamic covalent bond exchange reaction in a polymer network. In some way, this investigation follows the logic of a ‘solvent effect’ study that is typical for the development of an organic reaction or synthetic method in solution. Thus, this work constitutes a study of matrix effects on the viscoelastic properties of vitrimers, in particular with regard to their stress-relaxation behaviour. For that purpose, the dynamic transamination within vinylogous urethanes (VU) vitrimers, derived from a wide range of different commercially available diols, was chosen as vitrimer chemistry reaction platform. Additionally, the influence of the molecular weight, and thus the cross-link density, was also investigated using two oligomeric diols of different molecular weights. It was found that changing the molecular weight resulted in vitrimers with significantly different activation energies (from 68 to 149 kJ mol−1) and relaxation times (from 7 to 230 min at 140 °C). These remarkable results suggest that both the molecular weight and the nature of the polymer matrix have a large influence on the resulting viscoelastic properties.
Abstract Vitrimer materials are a rapidly growing field of research, in which still many fundamental aspects of material design remain to be explored. In this study, we report a systematic study about the effect of the choice of the matrix on a dynamic covalent bond exchange reaction in a polymer network. In some way, this investigation follows the logic of a ‘solvent effect’ study that is typical for the development of an organic reaction or synthetic method in solution. Thus, this work constitutes a study of matrix effects on the viscoelastic properties of vitrimers, in particular with regard to their stress-relaxation behaviour. For that purpose, the dynamic transamination within vinylogous urethanes (VU) vitrimers, derived from a wide range of different commercially available diols, was chosen as vitrimer chemistry reaction platform. Additionally, the influence of the molecular weight, and thus the cross-link density, was also investigated using two oligomeric diols of different molecular weights. It was found that changing the molecular weight resulted in vitrimers with significantly different activation energies (from 68 to 149 kJ mol−1) and relaxation times (from 7 to 230 min at 140 °C). These remarkable results suggest that both the molecular weight and the nature of the polymer matrix have a large influence on the resulting viscoelastic properties.
Stereocontrolled, multi-functional sequence-defined oligomers through automated synthesis
C. Mertens, M. Soete, M.L. Ślęczkowski, A.R.A. Palmans, E. W. Meijer, N. Badi, F.E. Du Prez
Polym. Chem., 2020
C. Mertens, M. Soete, M.L. Ślęczkowski, A.R.A. Palmans, E. W. Meijer, N. Badi, F.E. Du Prez
Polym. Chem., 2020
 Abstract In contrast to biomacromolecules, synthetic polymers generally lack a defined monomer sequence, therefore one of the challenges of polymer chemists these days is gaining more control over the primary structure of synthetic polymers and oligomers. In this work, stereocontrolled sequence-defined oligomers were synthesised using a thiolactone-based platform. Step-wise elongation of the oligomer occurs via ring-opening of the thiolactone, resulting in the formation of stereocenters along the backbone. These initial studies indicate remarkable differences in the strength of non-covalent interactions in isotactic and atactic oligomers. Different side-chain moieties were introduced using alkyl halide building blocks and the synthetic protocol was succesfully optimised and automated. Furthermore, the possible post-synthesis modification of the oligomers was demonstrated using ‘click’ chemistry.
Abstract In contrast to biomacromolecules, synthetic polymers generally lack a defined monomer sequence, therefore one of the challenges of polymer chemists these days is gaining more control over the primary structure of synthetic polymers and oligomers. In this work, stereocontrolled sequence-defined oligomers were synthesised using a thiolactone-based platform. Step-wise elongation of the oligomer occurs via ring-opening of the thiolactone, resulting in the formation of stereocenters along the backbone. These initial studies indicate remarkable differences in the strength of non-covalent interactions in isotactic and atactic oligomers. Different side-chain moieties were introduced using alkyl halide building blocks and the synthetic protocol was succesfully optimised and automated. Furthermore, the possible post-synthesis modification of the oligomers was demonstrated using ‘click’ chemistry.
Exploration of the Selectivity and Retention Behavior of Alternative Polyacrylamides in Temperature Responsive Liquid Chromatography
M. Baert, K. Wicht, Z. Hou, R. Szucs, F.E. Du Prez, F. Lynen
Anal. Chem., 2020
M. Baert, K. Wicht, Z. Hou, R. Szucs, F.E. Du Prez, F. Lynen
Anal. Chem., 2020
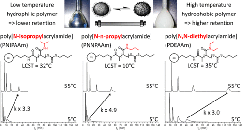 Abstract Temperature responsive liquid chromatography (TRLC) allows for separation of organic solutes in purely aqueous mobile phases whereby retention is controlled through temperature. The vast majority of the work has thus far been performed on poly[N-isopropylacrylamide] (PNIPAAm)-based columns, while the performance of other temperature responsive polymers has rarely been compared under identical conditions. Therefore, in this work, two novel TRLC phases based on poly[N-n-propylacrylamide] (PNNPAAm) and poly[N,N-diethylacrylamide] (PDEAAm) are reported and compared to the state of the art PNIPAAm based column. Optimal comparison is thereby obtained by the use of controlled radical polymerizations, identical molecular weights, and by maximizing carbon loads on the silica supporting material. Analysis of identical test mixtures of homologue series and pharmaceutical samples revealed that PNNPAAm performs in a similar way as PNIPAAm while offering enhanced retention and a shift of the useable temperature range toward lower temperatures. PDEAAm offers a range of novel possibilities as it depicts a different selectivity, allowing for enhanced resolution in TRLC in, for example, coupled column systems. Reduced plate heights of 3 could be obtained on the homemade columns, offering the promise for reasonable column efficiencies in TRLC despite the use of bulky polymers as stationary phases in HPLC.
Abstract Temperature responsive liquid chromatography (TRLC) allows for separation of organic solutes in purely aqueous mobile phases whereby retention is controlled through temperature. The vast majority of the work has thus far been performed on poly[N-isopropylacrylamide] (PNIPAAm)-based columns, while the performance of other temperature responsive polymers has rarely been compared under identical conditions. Therefore, in this work, two novel TRLC phases based on poly[N-n-propylacrylamide] (PNNPAAm) and poly[N,N-diethylacrylamide] (PDEAAm) are reported and compared to the state of the art PNIPAAm based column. Optimal comparison is thereby obtained by the use of controlled radical polymerizations, identical molecular weights, and by maximizing carbon loads on the silica supporting material. Analysis of identical test mixtures of homologue series and pharmaceutical samples revealed that PNNPAAm performs in a similar way as PNIPAAm while offering enhanced retention and a shift of the useable temperature range toward lower temperatures. PDEAAm offers a range of novel possibilities as it depicts a different selectivity, allowing for enhanced resolution in TRLC in, for example, coupled column systems. Reduced plate heights of 3 could be obtained on the homemade columns, offering the promise for reasonable column efficiencies in TRLC despite the use of bulky polymers as stationary phases in HPLC.
Shining Light on Poly(ethylene glycol): From Polymer Modification to 3D Laser Printing of Water Erasable Microstructures
H.A. Houck, P. Müller, M. Wegener, C. Barner‐Kowollik, F.E. Du Prez, E. Blasco
Adv. Mater., 2020
H.A. Houck, P. Müller, M. Wegener, C. Barner‐Kowollik, F.E. Du Prez, E. Blasco
Adv. Mater., 2020
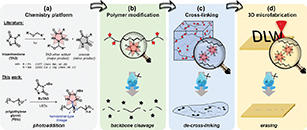 Abstract The implementation of stimuli‐responsive bonds into 3D network assemblies is a key concept to design adaptive materials that can reshape and degrade. Here, a straightforward but unique photoresist is introduced for the tailored fabrication of poly(ethylene glycol) (PEG) materials that can be readily erased by water, even without the need for acidic or basic additives. Specifically, a new class of photoresist is developed that operates through the backbone crosslinking of PEG when irradiated in the presence of a bivalent triazolinedione. Hence, macroscopic gels are obtained upon visible light‐emitting diode irradiation (λ > 515 nm) that are stable in organic media but rapidly degrade upon the addition of water. Photoinduced curing is also applicable to multiphoton laser lithography (λ > 700 nm), hence providing access to 3D printed microstructures that vanish when immersed in water at 37 °C. Materials with varying crosslinking densities are accessed by adapting the applied laser writing power, thereby allowing for tunable hydrolytic erasing timescales. A new platform technology is thus presented that enables the crosslinking and 3D laser printing of PEG‐based materials, which can be cleaved and erased in water, and additionally holds potential for the facile modification and backbone degradation of polyether‐containing materials in general.
Abstract The implementation of stimuli‐responsive bonds into 3D network assemblies is a key concept to design adaptive materials that can reshape and degrade. Here, a straightforward but unique photoresist is introduced for the tailored fabrication of poly(ethylene glycol) (PEG) materials that can be readily erased by water, even without the need for acidic or basic additives. Specifically, a new class of photoresist is developed that operates through the backbone crosslinking of PEG when irradiated in the presence of a bivalent triazolinedione. Hence, macroscopic gels are obtained upon visible light‐emitting diode irradiation (λ > 515 nm) that are stable in organic media but rapidly degrade upon the addition of water. Photoinduced curing is also applicable to multiphoton laser lithography (λ > 700 nm), hence providing access to 3D printed microstructures that vanish when immersed in water at 37 °C. Materials with varying crosslinking densities are accessed by adapting the applied laser writing power, thereby allowing for tunable hydrolytic erasing timescales. A new platform technology is thus presented that enables the crosslinking and 3D laser printing of PEG‐based materials, which can be cleaved and erased in water, and additionally holds potential for the facile modification and backbone degradation of polyether‐containing materials in general.
Introduction to chemistry for covalent adaptable networks
C. Bowman, F. Du Prez, J. Kalow
Polym. Chem., 2020
C. Bowman, F. Du Prez, J. Kalow
Polym. Chem., 2020
 Abstract Christopher Bowman, Filip Du Prez and Julia Kalow introduce the Polymer Chemistry themed collection on chemistry for covalent adaptable networks.
Abstract Christopher Bowman, Filip Du Prez and Julia Kalow introduce the Polymer Chemistry themed collection on chemistry for covalent adaptable networks.
On-Demand Dissoluble Diselenide-Containing Hydrogel
W. Lu, X. Xu, L. Imbernon, J. Zhu, R. Hoogenboom, F.E. Du Prez, X. Pan
Biomac., 2020
W. Lu, X. Xu, L. Imbernon, J. Zhu, R. Hoogenboom, F.E. Du Prez, X. Pan
Biomac., 2020
 Abstract On-demand dissolution of hydrogels is being increasingly studied for their potential use in burn wound dressing applications. Herein, a dynamic diselenide-containing hydrogel is developed through a very simple one-pot and two-step process starting from the selenol functionalization of a partially hydrolyzed poly(2-ethyl-2-oxazoline) with γ-butyroselenolactone. The hydrogel spontaneously cross-links via an in situ oxidation of the selenol functionalities in air. The gelation process and the final viscoelastic properties of the gel are characterized by rheological experiments. The mechanical properties of those new diselenide-containing hydrogels are easily tuned by varying the concentration of γ-butyroselenolactone. The materials also show good skin adhesion and UV light responsiveness. A unique feature of the hydrogel is its capability to be fully and rapidly dissolved on-demand, via oxidation or reduction of the diselenide cross-links, making them particularly attractive for burn wound dressing applications.
Abstract On-demand dissolution of hydrogels is being increasingly studied for their potential use in burn wound dressing applications. Herein, a dynamic diselenide-containing hydrogel is developed through a very simple one-pot and two-step process starting from the selenol functionalization of a partially hydrolyzed poly(2-ethyl-2-oxazoline) with γ-butyroselenolactone. The hydrogel spontaneously cross-links via an in situ oxidation of the selenol functionalities in air. The gelation process and the final viscoelastic properties of the gel are characterized by rheological experiments. The mechanical properties of those new diselenide-containing hydrogels are easily tuned by varying the concentration of γ-butyroselenolactone. The materials also show good skin adhesion and UV light responsiveness. A unique feature of the hydrogel is its capability to be fully and rapidly dissolved on-demand, via oxidation or reduction of the diselenide cross-links, making them particularly attractive for burn wound dressing applications.
Thermal dissociation of anthracene photodimers in the condensed state: kinetic evaluation and complex phase behaviour
J. Brancart, J. Van Damme, F.E. Du Prez, G. Van Assche
Phys. Chem. Chem. Phys., 2020
J. Brancart, J. Van Damme, F.E. Du Prez, G. Van Assche
Phys. Chem. Chem. Phys., 2020
 Abstract Thermally and photochemically reversible functional groups, such as photodimers of anthracene derivatives, offer interesting stimuli-responsive behaviour. To evaluate their potential for application in reversible polymer networks, accurate kinetic parameters and knowledge of their thermophysical behaviour are required. Accurate kinetic studies of the thermal dissociation of the photodimers in the condensed state, thus without the influence of solvents on their reactivity, is still lacking. A methodology was set up to accurately evaluate the chemical reaction kinetics and complex phase behaviour during the thermal dissociation of photodimers into their corresponding monomers. Temperature-controlled time-resolved FTIR spectroscopy was used to determine the reaction progress, while non-isothermal DSC measurements were used to study the thermophysical changes, resulting from the thermal dissociation reaction. The thermal dissociation behaviour in the condensed state is more challenging than in the solution state due to the crystallinity of the dimers, stabilizing the dimers and thus slowing down the initial dissociation rates. Distinctly different sets of kinetic parameters were found for the dissociation from the molten and the crystalline state. For experiments performed below the melting temperature of the photodimer, the reaction rate changes abruptly as the dimer is partly dissociated and partly dissolved into the formed monomer...
Abstract Thermally and photochemically reversible functional groups, such as photodimers of anthracene derivatives, offer interesting stimuli-responsive behaviour. To evaluate their potential for application in reversible polymer networks, accurate kinetic parameters and knowledge of their thermophysical behaviour are required. Accurate kinetic studies of the thermal dissociation of the photodimers in the condensed state, thus without the influence of solvents on their reactivity, is still lacking. A methodology was set up to accurately evaluate the chemical reaction kinetics and complex phase behaviour during the thermal dissociation of photodimers into their corresponding monomers. Temperature-controlled time-resolved FTIR spectroscopy was used to determine the reaction progress, while non-isothermal DSC measurements were used to study the thermophysical changes, resulting from the thermal dissociation reaction. The thermal dissociation behaviour in the condensed state is more challenging than in the solution state due to the crystallinity of the dimers, stabilizing the dimers and thus slowing down the initial dissociation rates. Distinctly different sets of kinetic parameters were found for the dissociation from the molten and the crystalline state. For experiments performed below the melting temperature of the photodimer, the reaction rate changes abruptly as the dimer is partly dissociated and partly dissolved into the formed monomer...
Double neighbouring group participation for ultrafast exchange in phthalate monoester networks
M. Delahaye, F. Tanini, J.O. Holloway, J.M. Winne, F.E. Du Prez
Polym. Chem., 2020
M. Delahaye, F. Tanini, J.O. Holloway, J.M. Winne, F.E. Du Prez
Polym. Chem., 2020
 Abstract Phthalate monoesters (PMEs) were recently introduced as a simple dynamic covalent bond for implementation in covalent adaptable networks (CANs), which undergo rapid transesterifications in the absence of catalysts, due to the neighbouring group participation (NGP) of a carboxylic acid moiety. In this work, it is shown that the PME transesterification can be very significantly accelerated by the presence of another neighbouring group on the reactive alcohol moieties. The kinetic effects are demonstrated using a short model study of PMEs with different substituents at the β-carbon position, showing a remarkable acceleration for alcohols containing tertiary amines on the β-carbon. Following the model study, materials were synthesised by a (partial) replacement of the conventionally used diol with a β-amino-diol, leading to the formation of networks with an increased Tg and Young's-modulus, which is rationalised as a result of the formation of an ionic network (COO− and NHR3+). Stress relaxation experiments show a decrease in relaxation times by a factor of 500, compared to similar networks derived from non-amine-substituted hydroxyl monomers. This ultrafast relaxation, enabled by a double NGP, resulted in CANs that show potential to be processed through extrusion while maintaining their overall network connectivity.
Abstract Phthalate monoesters (PMEs) were recently introduced as a simple dynamic covalent bond for implementation in covalent adaptable networks (CANs), which undergo rapid transesterifications in the absence of catalysts, due to the neighbouring group participation (NGP) of a carboxylic acid moiety. In this work, it is shown that the PME transesterification can be very significantly accelerated by the presence of another neighbouring group on the reactive alcohol moieties. The kinetic effects are demonstrated using a short model study of PMEs with different substituents at the β-carbon position, showing a remarkable acceleration for alcohols containing tertiary amines on the β-carbon. Following the model study, materials were synthesised by a (partial) replacement of the conventionally used diol with a β-amino-diol, leading to the formation of networks with an increased Tg and Young's-modulus, which is rationalised as a result of the formation of an ionic network (COO− and NHR3+). Stress relaxation experiments show a decrease in relaxation times by a factor of 500, compared to similar networks derived from non-amine-substituted hydroxyl monomers. This ultrafast relaxation, enabled by a double NGP, resulted in CANs that show potential to be processed through extrusion while maintaining their overall network connectivity.
Vitrimers: directing chemical reactivity to control material properties
M. Guerre, C. Taplan, J.M. Winne, F.E. Du Prez
Chem. Sci., 2020
M. Guerre, C. Taplan, J.M. Winne, F.E. Du Prez
Chem. Sci., 2020
 Abstract The development of more sustainable materials with a prolonged useful lifetime is a key requirement for a transition towards a more circular economy. However, polymer materials that are long-lasting and highly durable also tend to have a limited application potential for re-use. This is because such materials derive their durable properties from a high degree of chemical connectivity, resulting in rigid meshes or networks o polymer chains with a high intrinsic resistance to deformation. Once such polymers are fully synthesized, thermal (re)processing becomes hard (or impossible) to achieve without damaging the degree of chemical connectivity, and most recycling options quickly lead to a drop or even loss of material properties. In this context, both academic and industrial researchers have taken a keen interest in materials design that combine high degrees of chemical connectivity with an improved thermal (re)processability, mediated through a dynamic exchange reaction of covalent bonds. In particular vitrimer materials offer a promising concept because they completely maintain their degree of chemical connectivity at all times, yet can show a clear thermally driven plasticity and liquid behavior, enabled through rapid bond rearrangement reactions within the network...
Abstract The development of more sustainable materials with a prolonged useful lifetime is a key requirement for a transition towards a more circular economy. However, polymer materials that are long-lasting and highly durable also tend to have a limited application potential for re-use. This is because such materials derive their durable properties from a high degree of chemical connectivity, resulting in rigid meshes or networks o polymer chains with a high intrinsic resistance to deformation. Once such polymers are fully synthesized, thermal (re)processing becomes hard (or impossible) to achieve without damaging the degree of chemical connectivity, and most recycling options quickly lead to a drop or even loss of material properties. In this context, both academic and industrial researchers have taken a keen interest in materials design that combine high degrees of chemical connectivity with an improved thermal (re)processability, mediated through a dynamic exchange reaction of covalent bonds. In particular vitrimer materials offer a promising concept because they completely maintain their degree of chemical connectivity at all times, yet can show a clear thermally driven plasticity and liquid behavior, enabled through rapid bond rearrangement reactions within the network...
Dynamic Curing Agents for Amine-Hardened Epoxy Vitrimers with Short (Re)processing Times
Y. Spiesschaert, M. Guerre, I. De Baere, W. Van Paepegem, J.M. Winne, F.E. Du Prez
Macromol., 53 (7), 2485-2495, 2020
Y. Spiesschaert, M. Guerre, I. De Baere, W. Van Paepegem, J.M. Winne, F.E. Du Prez
Macromol., 53 (7), 2485-2495, 2020
 Abstract This work presents a straightforward strategy to introduce highly dynamic and adaptable cross-links into common epoxy resin formulations. For this, an oligomeric amine-based curing agent containing vinylogous urethane (VU) bonds was developed. This novel polyfunctional amine curing agent can be used as a drop-in solution for existing epoxy resin technologies, resulting in transparent, rigid, and, at the same time, highly reprocessable catalyst-free epoxy vitrimers. The oligomeric VU-curing agents are prepolymerized via a straightforward condensation reaction between acetoacetates extended with different classical amine monomers and epoxy hardeners. It is found that vitrimer properties can be readily introduced into these epoxy formulations by converting <50 mol % of the hardener’s amine functionalities into dynamic vinylogous urethane bonds. In this way, epoxy vitrimers can be obtained with material properties comparable to ones of the VU-free epoxy formulations. In addition, remarkably short processing times are observed in the absence of any catalyst, and the material displayed very short stress relaxation times and good recyclability, actually representing the most performant VU-based vitrimers so far. Furthermore, a proof of concept for its use in obtaining glass-fiber-reinforced epoxy composites is also presented.
Abstract This work presents a straightforward strategy to introduce highly dynamic and adaptable cross-links into common epoxy resin formulations. For this, an oligomeric amine-based curing agent containing vinylogous urethane (VU) bonds was developed. This novel polyfunctional amine curing agent can be used as a drop-in solution for existing epoxy resin technologies, resulting in transparent, rigid, and, at the same time, highly reprocessable catalyst-free epoxy vitrimers. The oligomeric VU-curing agents are prepolymerized via a straightforward condensation reaction between acetoacetates extended with different classical amine monomers and epoxy hardeners. It is found that vitrimer properties can be readily introduced into these epoxy formulations by converting <50 mol % of the hardener’s amine functionalities into dynamic vinylogous urethane bonds. In this way, epoxy vitrimers can be obtained with material properties comparable to ones of the VU-free epoxy formulations. In addition, remarkably short processing times are observed in the absence of any catalyst, and the material displayed very short stress relaxation times and good recyclability, actually representing the most performant VU-based vitrimers so far. Furthermore, a proof of concept for its use in obtaining glass-fiber-reinforced epoxy composites is also presented.
From Sequence‐Defined Macromolecules to Macromolecular Pin Codes
J.O. Holloway, F. Van Lijsebetten, N. Badi, H.A. Houck, F.E. Du Prez
Adv. Sci., 1903698, 2020
J.O. Holloway, F. Van Lijsebetten, N. Badi, H.A. Houck, F.E. Du Prez
Adv. Sci., 1903698, 2020
2020.jpg) Abstract Dynamic sequence‐defined oligomers carrying a chemically written pin code are obtained through a strategy combining multicomponent reactions with the thermoreversible addition of 1,2,4‐triazoline‐3,5‐diones (TADs) to indole substrates. The precision oligomers are specifically designed to be encrypted upon heating as a result of the random reshuffling of the TAD‐indole covalent bonds within the backbone, thereby resulting in the scrambling of the encoded information. The encrypted pin code can eventually be decrypted following a second heating step that enables the macromolecular pin code to be deciphered using 1D electrospray ionization‐mass spectrometry (ESI‐MS). The herein introduced concept of encryption/decryption represents a key advancement compared with current strategies that typically use uncontrolled degradation to erase and tandem mass spectrometry (MS/MS) to analyze, decipher, and read‐out chemically encrypted information. Additionally, the synthesized macromolecules are coated onto a high‐value polymer material, which demonstrates their potential application as coded product tags for anti‐counterfeiting purposes.
Abstract Dynamic sequence‐defined oligomers carrying a chemically written pin code are obtained through a strategy combining multicomponent reactions with the thermoreversible addition of 1,2,4‐triazoline‐3,5‐diones (TADs) to indole substrates. The precision oligomers are specifically designed to be encrypted upon heating as a result of the random reshuffling of the TAD‐indole covalent bonds within the backbone, thereby resulting in the scrambling of the encoded information. The encrypted pin code can eventually be decrypted following a second heating step that enables the macromolecular pin code to be deciphered using 1D electrospray ionization‐mass spectrometry (ESI‐MS). The herein introduced concept of encryption/decryption represents a key advancement compared with current strategies that typically use uncontrolled degradation to erase and tandem mass spectrometry (MS/MS) to analyze, decipher, and read‐out chemically encrypted information. Additionally, the synthesized macromolecules are coated onto a high‐value polymer material, which demonstrates their potential application as coded product tags for anti‐counterfeiting purposes.
Mesoporous TiO2 from poly(N,N-dimethylacrylamide)-b-polystyrene block copolymers for long-term acetaldehyde photodegradation
J. Billet, S. Vandewalle, M. Meire, N. Blommaerts, P. Lommens, S. Verbruggen, K. De Buysser,
F.E. Du Prez, I. Van Driessche
J. Mater. Sci., 55, 1933-1945, 2020
J. Billet, S. Vandewalle, M. Meire, N. Blommaerts, P. Lommens, S. Verbruggen, K. De Buysser,
F.E. Du Prez, I. Van Driessche
J. Mater. Sci., 55, 1933-1945, 2020
 Abstract Although already some mesoporous (2–50 nm) sol–gel TiO2 synthesis strategies exist, no pore size control beyond the 12 nm range is possible without using specialized organic structure-directing agents synthetized via controlled anionic/radical polymerizations. Here, we present the use of reversible addition–fragmentation chain transfer (RAFT) polymerization as a straightforward and industrial applicable alternative to the existing controlled polymerization methods for structure-directing agent synthesis. Poly(N,N-dimethylacrylamide)-block-polystyrene (PDMA-b-PS) block copolymer, synthesized via RAFT, was chosen as structure-directing agent for the formation of the mesoporous TiO2. Crack-free thin layers TiO2 with tunable pores from 8 to 45 nm could be acquired. For the first time, in a detailed and systematic approach, the influence of the block size and dispersity of the block copolymer is experimentally screened for their influence on the final meso-TiO2 layers. As expected, the mesoporous TiO2 pore sizes showed a clear correlation to the polystyrene block size and the dispersity of the PDMA-b-PS block copolymer. Surprisingly, the dispersity of the polymer was shown not to be affecting the standard deviation of the pores...
Abstract Although already some mesoporous (2–50 nm) sol–gel TiO2 synthesis strategies exist, no pore size control beyond the 12 nm range is possible without using specialized organic structure-directing agents synthetized via controlled anionic/radical polymerizations. Here, we present the use of reversible addition–fragmentation chain transfer (RAFT) polymerization as a straightforward and industrial applicable alternative to the existing controlled polymerization methods for structure-directing agent synthesis. Poly(N,N-dimethylacrylamide)-block-polystyrene (PDMA-b-PS) block copolymer, synthesized via RAFT, was chosen as structure-directing agent for the formation of the mesoporous TiO2. Crack-free thin layers TiO2 with tunable pores from 8 to 45 nm could be acquired. For the first time, in a detailed and systematic approach, the influence of the block size and dispersity of the block copolymer is experimentally screened for their influence on the final meso-TiO2 layers. As expected, the mesoporous TiO2 pore sizes showed a clear correlation to the polystyrene block size and the dispersity of the PDMA-b-PS block copolymer. Surprisingly, the dispersity of the polymer was shown not to be affecting the standard deviation of the pores...
Fast processing of highly crosslinked, low-viscosity vitrimers
C. Taplan, M. Guerre, J.M. Winne, F.E. Du Prez
Mater. Horiz., 7, 104-110, 2020
C. Taplan, M. Guerre, J.M. Winne, F.E. Du Prez
Mater. Horiz., 7, 104-110, 2020
 Abstract Here we describe a rational approach to go beyond the current processability limits of vitrimer materials, with a demonstration of low-viscosity fast processing of highly crosslinked permanent networks. Vitrimers are a recently introduced class of polymer networks with a unique glass-like viscoelastic behavior, in which bond exchange reactions govern the macroscopic material flow. The restricted chain mobility, only enabled by chemical exchanges, typically limits the use of continuous processing techniques, such as extrusion or injection moulding. Herein, we outline a straightforward materials design approach, taking into account both the effect of minor additives on the chemistry of bond rearrangement as well as the macromolecular architecture of the vitrimeric network. These combined effects are demonstrated to work in an additive fashion, culminating in stress relaxation times below 1 s at 150 °C. The observed rapid bond exchanges in permanent networks result in an unprecedented control of the polymer material behavior, where the material flow is still dominated by chemical exchanges, but only marginally limited by the chemical exchange rate, overcoming the challenges encountered so far in continuous processing of highly crosslinked vitrimeric systems.
Abstract Here we describe a rational approach to go beyond the current processability limits of vitrimer materials, with a demonstration of low-viscosity fast processing of highly crosslinked permanent networks. Vitrimers are a recently introduced class of polymer networks with a unique glass-like viscoelastic behavior, in which bond exchange reactions govern the macroscopic material flow. The restricted chain mobility, only enabled by chemical exchanges, typically limits the use of continuous processing techniques, such as extrusion or injection moulding. Herein, we outline a straightforward materials design approach, taking into account both the effect of minor additives on the chemistry of bond rearrangement as well as the macromolecular architecture of the vitrimeric network. These combined effects are demonstrated to work in an additive fashion, culminating in stress relaxation times below 1 s at 150 °C. The observed rapid bond exchanges in permanent networks result in an unprecedented control of the polymer material behavior, where the material flow is still dominated by chemical exchanges, but only marginally limited by the chemical exchange rate, overcoming the challenges encountered so far in continuous processing of highly crosslinked vitrimeric systems.
- Molecular access to multi-dimensionally encoded information, J. Steinkoenig, R. Aksakal, F.E. Du Prez, Eur. Polym. J., 120, 109260, 2019
- Covalent Adaptable Networks with Tunable Exchange Rates Based on Reversible Thiol–yne Cross‐Linking, N. Van Herck, D. Maes, K. Unal, M. Guerre, J. M. Winne, F.E. Du Prez, Angew. Chem., DOI: 10.1002/anie.201912902, 2019
- Dynamic Covalent Chemistry in Polymer Networks: A Mechanistic Perspective, J.M. Winne, L. Leibler, F.E. Du Prez, Polym. Chem., 10, 6091-6108, 2019
- Internal Catalysis in Covalent Adaptable Networks: Phthalate Monoester Transesterification As a Versatile Dynamic Cross-Linking Chemistry, M. Delahaye, J.M. Winne, F.E. Du Prez, J. Am. Chem. Soc., 141, 15277-15287, 2019
- Digging into the Sequential Space of Thiolactone Precision Polymers: A Combinatorial Strategy to Identify Functional Domains, S. Celasun, D. Remmler, T. Schwaar,M.G. Weller, F.E. Du Prez, H.G. Börner, Hans., Angew. Chem., 58 (7), 1960-1964, 2019
- Urethane polythioether self-crosslinking resins, B. Hendriks, O. van den Berg, F.E. Du Prez, Progress in Organic Coatings, 136, 2019
- Full and Partial Amidation of Poly(methyl acrylate) as Basis for Functional Polyacrylamide (Co)Polymers, J. Van Guyse, J. Verjans, S. Vandewalle, K. De Bruycker, F.E. Du Prez, R. Hoogenboom, Macromol., 52 (14), 5102-5109, 2019
- Light-Stabilised Dynamic Materials, H.A. Houck, E. Blasco, F.E. Du Prez, C. Barner-Kowollik,, J. Am. Chem. Soc., 141, 12329-12337, 2019
- Direct Comparison of Solution and Solid Phase Synthesis of Sequence-Defined Macromolecules, J.O. Holloway, K.S. Wetzel, S. Martens, F.E. Du Prez, M.A. R. Meier, Polym. Chem., 10, 3859-3867, 2019
- Multi-olefin containing polyethers and triazolinediones: a powerful alliance, T. Johann, H.A. Houck, T. Dinh, U. Kemmer-Jonas, F.E. Du Prez, H. Frey, Polym. Chem., 10, 4699-4708, 2019
- Sustainable synthesis of renewable terpenoid-based (meth)acrylates using the CHEM21 green metrics toolkit, M.A. Droesbeke, F.E. Du Prez, ACS Sustainable Chem. Eng., 7, 11633-11639, 2019
- Filler reinforced polydimethylsiloxane-based vitrimers, Y. Spiesschaert, M. Guerre, L. Imbernon, J.M. Winne, F.E. Du Prez, Polymer, 172, 239-246, 2019
- TAD Click Chemistry on Aliphatic Polycarbonates: A First Step Toward Tailor-Made Materials, A. Baroni, L. Vlaminck, L. Mespouille, F.E. Du Prez, N. Delbosc, B. Blankert, Macromol. Rapid Commun., 40, 1800743, 2019
- Conformational influence of fluorinated building blocks on the physical properties of polyesters, S. Lingier, R. Szpera, B. Goderis, B. Linclau, F.E. Du Prez, Polymer, 164, 134-141, 2019
- A novel donor-π-acceptor anthracene monomer: Towards faster and milder reversible dimerization, J. Van Damme, O. van den Berg, J. Brancart, G. Van Assche, F.E. Du Prez, Tetrahedron, 75, 912-920, 2019
- Thiolactone Chemistry for the Synthesis of Functional Silicone-Based Amphiphilic Co-Networks, K. De Bruycker, C. Mertens, F.E. Du Prez, J. Polym. Sci. A, 57(3), 322-333, 2019
- Thermoplastic polyacetals: Chemistry from the past for a sustainable future?, A. Hufendiek, S. Lingier, F.E. Du Prez, Polym. Chem., 10(1), 9-33, 2019
- Polycycloacetals via Polytransacetalization of Diglycerol Bisacetonide, A. Hufendiek, S. Lingier, P. Espeel, S. De Wildeman, F.E. Du Prez, Polym. Chem., 9, 4789-4797 , 2018
- In situ crosslinked nanofibers by aqueous electrospinning of selenol-functionalized poly(2-oxazoline)s, Y. Li, M. Vergaelen, X. Pan, F.E. Du Prez, R. Hoogenboom, K. De Clerck, Macromolecules, 51, 6149−6156, 2018
- Double-modified Glycopolymers from Thiolactones to Modulate Lectin Selectivity and Affinity, L.E. Wilkins, N. Badi, F.E. Du Prez, M.I. Gibson, ACS Macro Lett., 7, 1498−1502, 2018
- Automated Synthesis Protocol of Sequence-Defined Oligo-Urethane-Amides Using Thiolactone Chemistry, J.O. Holloway, C. Mertens, F.E. Du Prez,N. Badi, Macromol. Rapid Commun., 40, 1800685, 2018
- Multifunctional sequence-defined macromolecules for chemical data storage, S. Martens, A. Landuyt, P. Espeel, B. Devreese, P. Dawyndt, F.E. Du Prez, Nat.Commun., 9, 4451, 2018
- Polycaprolactone-b-poly(N-isopropylacrylamide) nanoparticles : synthesis and temperature induced coacervation behavior, S. Vandewalle, M. Van De Walle, S. Chattopadhyay, F.E. Du Prez, Eur. Polym. J., 98, 468-474, 2018
- Fluorinated vitrimer elastomers with a dual temperature response, M. Guerre, C. Taplan, R. Nicolay, J. Winne, F.E. Du Prez, J. Am. Chem. Soc., 140, 13272-13284, 2018
- Dynamic Diselenide-Containing Polyesters from Alcoholysis/Oxidation of γ-Butyroselenolactone, C. Wang, X. An, M. Pang, Z. Zhang, X. Zhu, J. Zhu, F.E. Du Prez, X. Pan, Polym. Chem., 9, 4044-4051, 2018
- Anthracene-based polyurethane networks: Tunable thermal degradation, photochemical cure and stress-relaxation, J. Van Damme, O. van den Berg, J. Brancart, G. Van Assche, F.E. Du Prez, Eur.Polym.J., 105, 412-420, 2018
- Fast Healing of Polyurethane Thermosets Using Reversible Triazolinedione Chemistry and Shape-Memory, N. Van Herck, F.E. Du Prez, Macromolecules, 51, 3405−3414, 2018
- Anthracene‐Based Colloidal Polymer Nanoparticles: Their Photochemical Ligation and Waterborne Coating Applications, S. Chattopadhyay,J. Van Damme, O. van den Berg, F.E. Du Prez, Part. Part. Syst. Charact., 35, 1800030, 2018
- Bifunctionalized redox-responsive layers prepared from a thiolactone copolymer, C. Chattaway, S. Belbekhouche,F.E. Du Prez, K. Glinel, S. Madeleine Demoustier-Champagne, Langmuir, 34, 5234–5244, 2018
- Tunable Blocking Agents for Temperature-Controlled Triazolinedione-Based Cross-Linking Reactions, H.A. Houck, K. De Bruycker, C. Barner-Kowollik, J.M. Winne,F.E. Du Prez, Macromolecules, 51, 3156−3164, 2018
- Enhancing the possibilities of comprehensive two-dimensional liquid chromatography through hyphenation of purely aqueous temperature-responsive and reversed-phase liquid chromatography, M. Baert, S.Martens, G. Desmet, A.J. de Villiers, F.E. Du Prez, F. Lynen, Anal. Chem., 90 , 4961-4967, 2018
- Anthracene-containing polymers toward high-end applications, J. Van Damme, F.E. Du Prez, Prog. Polym. Sci., 82, 92–119, 2018
- Vinylogous Urea Vitrimers and Their Application in Fiber Reinforced Composites, W. Denissen, I. De Baere, W. Van Paepegem, L. Leibler, J.M. Winne, F.E. Du Prez, Macromolecules, 51(5), 2054-2064, 2018
- Ultrafast Tailoring of Carbon Surfaces via Electrochemically Attached Triazolinediones, W. Laure, K. De Bruycker, P. Espeel, D. Fournier, P. Woisel, F.E. Du Prez, J. Lyskawa, Langmuir, 34(7), 2397–2402, 2018
- Structurally diverse polymers from norbornene and thiolactone containing building blocks, D. Frank, P. Espeel, N.Badi, F.E. Du Prez, Eur. Polym. J., 98, 246-253, 2018
- A Thiolactone Strategy for Straightforward Synthesis of Disulfide-Linked Side-Chain-to-Tail Cyclic Peptides Featuring an N-Terminal Modification Handle, D. Van Lysebetten, S. Felissati, E. Antonatou, L. Carrette, P. Espeel, E. Foquet, F.E. Du Prez, A. Madder, ChemBioChem., 19(6), 641-646, 2018
- Tyrosine-Triazolinedione Bioconjugation as Site-Selective Protein Modification Starting from RAFT-Derived Polymers, S. Vandewalle, R. De Coen, B.G. De Geest, F.E. Du Prez, ACS Macro Lett., 6 (12), 1368–1372, 2017
- Controlling thermal reactivity with different colors of light, H.A. Houck,F.E. Du Prez, C. Barner-Kowollik, Nat. Com., DOI: 10.1038/s41467-017-02022-0, 2017
- High-Throughput Platform for Synthesis of Melamine-Formaldehyde Microcapsules, S. Çakir, E. Bauters, G. Rivero,T. Parasote, J. Paul, F.E. Du Prez, ACS Comb. Sci., 19, 447−454, 2017
- Precisely Alternating Functionalized Polyampholytes Prepared in a Single Pot from Sustainable Thiolactone Building Blocks, C. Resetco, D. Frank, N. U. Kaya, N. Badi, F.E. Du Prez, ACS Macrolett., 6, 277−280, 2017
- Rigid polyurethanes, polyesters and polycarbonates from renewable ketal monomers, S. Lingier, Y. Spiesschaert, B. Dhanis, S. De Wildeman, F.E. Du Prez, Macromolecules, 50, 5346–5352, 2017
- Combining Two Methods of Sequence Definition in a Convergent Approach: Scalable Synthesis of Highly Defined, Multifunctionalized Macromolecules, S. C. Solleder, S.P. Martens, P. Espeel, F.E. Du Prez, M.A.R. Meier, Chem. Eur. J., 23, 1–5, 2017
- Polyamides based on a partially bio-based spirodiamine, A. Wroblewska, S. Lingier, J. Noordijk, F.E. Du Prez, S. De Wildeman, K. Bernaerts, Eur. Polym. J., 96, 221-231, 2017
- Preparation of Janus nanoparticles from block copolymer thin films using triazolinedione chemistry, E. Poggi, W. Ouvry, B. Ernould, J.-P. Bourgeois, S. Chattopadhyay, F.E. Du Prez, J.-F. Gohy, RSC Adv., 7, 37048–37054, 2017
- Click and click-inspired chemistry for the design of sequence-controlled polymers, S. Martens, J.O. Holloway, F.E. Du Prez, Macromol. Rapid Commun., 38, 1700469, 2017
- Triazolinedione-“clicked” poly(phosphoester)s: Systematic adjustment of thermal properties, G. Becke, L. Vlaminck, M.M. Velencoso, F.E. Du Prez, F.R. Wurm, Polym. Chem., 8, 4074–4078, 2017
- Thiol-ene chemistry for polymer coatings and surface modification – building in sustainability and performance, C. Resetco, B. Hendriks, N. Badi, F.E. Du Prez, Mat. Horizons, 4, 1041-1053, 2017
- Lignin inspired phenolic polyethers synthesized via ADMET: Systematic structure-property investigation, L. Vlaminck, S. Lingier, A. Hufendiek, F.E. Du Prez, Eur. Polym. J., 95, 503-513, 2017
- PEGylated precision segments based on sequence-defined thiolactone oligomers, S. Celasu, F.E. Du Prez, H.G. Börner, Macromol. Rapid Commun., 38, 1700688, 2017
- Polydimethylsiloxane quenchable vitrimers, T. Stukenbroeker, W. Wang, J. Winne, F.E. Du Prez, R. Nicolaÿ, L. Leibler, Polym. Chem., 8, 6590-6593, 2017
- Sustainable synthesis routes towards urazole compounds, L. Vlaminck, B. Van de Voorde, F.E. Du Prez, Green Chem., 19, 5659-5664, 2017
- Tailored modification of thioacrylates in a versatile, sequence-defined procedure, J.O. Holloway, S. Aksakal, F.E. Du Prez, C.R. Becer, Macromol. Rapid Commun., 38, 1700500, 2017
- Covalent fluorination strategies for the surface modification of polydienes, K. De Bruycker, M. Delahaye, P. Cools, J.M. Winne, F.E. Du Prez, Macromol. Rapid Commun., 38, 1700122, 2017
- Poly(thioether) Vitrimers via Transalkylation of Trialkylsulfonium Salts, B. Hendriks, J. Waelkens,, J.M. Winne, F.E. Du Prez, ACS Macro Lett., 6, 930−934, 2017
- Chemical control of the viscoelastic properties of vinylogous urethane vitrimers, W. Denissen, M. Droesbeke, R. Nicolaÿ, L. Leibler, J. Winne, F.E. Du Prez, Nat. Commun., 8, 14857-14863, 2017
- Design of a thermally controlled sequence of triazolinedione-based click and transclick reactions, H.A. Houck, K. De Bruycker, S. Billiet, B. Dhanis, H. Goossens, S. Catak, V. Van Speybroeck, J.M. Winne, F.E. Du Prez, Chem. Sci., 8, 3098–3108, 2017
- Multifunctional Dendrimer Formation Using Thiolactone Chemistry, N. Uğur Kaya, F.E. Du Prez, N. Badi, , Macromol. Chem. Phys., 218, 1600575, 2017
- Selenolactone as Building Block towards Dynamic Diselenide Containing Polymer Architectures with Controllable Topology, X. Pan, F. Driessen, X. Zhu, F.E. Du Prez, ACS Macrolett., 6, 89−92, 2017
- Thiol-Michael Addition in Polar Aprotic Solvents: Nucleophilic Initiation or Base Catalysis?, G.B. Desmet, M.K. Sabbe, D.R. D’hooge, P. Espeel, S. Celasun, G.B. Marin, F.E. Du Prez, M-F. Reyniers, Polym. Chem., 8, 1341-1352, 2017
- Protected Thiol Strategies in Macromolecular Design, F. Goethals, D. Frank, F.E. Du Prez, Prog. Polym. Sci., 64, 76–113, 2017
- Immobilization of 2-deoxy-D-ribose-5-phosphate aldolase in polymeric thin films via the Langmuir-Schaefer technique, S. Reinicke, H.C. Rees, P. Espeel, N. Vanparijs, C. Bisterfeld, M. Dick, R.R. Rosencrantz, G. Brezesinski, B.G. De Geest, F.E. Du Prez, J. Pietruszka, A. Böker, ACS Appl. Mater. Interfaces, 9, 8317-8326, 2017
- Easy access to triazolinedione-endcapped peptides for chemical ligation, P. Wilke, T. Kunde, S. Chattopadhyay, N. ten Brummelhuis, F.E. Du Prez, H.G. Börner, Chem. Comm., 53, 593-596, 2017
- UV-cured multifunctional coating resins prepared from renewable thiolactone derivatives, C.Resetco, T. Dikić, T. Verbrugge, F.E. Du Prez, Prog. Org. Coatings, 107, 75-82, 2017
- Biodegradable polymer networks by the combination of oleyl-functionalized poly(-caprolactone) and TAD chemistry, M. Basko, M. Bednarek, L. Vlaminck, F.E. Du Prez, Eur. Polym. J., 89, 230–240, 2017
- Anthracene-based Thiol-ene Networks with Thermo-Degradable and Photo-Reversible properties J. Van Damme, O. van den Berg, J. Brancart, L. Vlaminck, C. Huyck, G. Van Assche, B. Van Mele, F.E. Du Prez, Macromolecules, 50, 1930-1938, 2017
- Responsive thiolactone-derived N-substituted poly(urethane-amide)s, P. Espeel, S. Celasun, P.S. Omurtag, S. Martens, F.E. Du Prez, Macrom. Rapid Comm., 38, 1600783, 2017
- Acrylate-based coatings to protect lead substrates, M. De Keersmaecker, T. Haufmann, O. van den Berg, S. Vandewalle, T. Muselle, K. Verbeken, A. Hubin, F.E. Du Prez, A. Adriaens, Electrochimica Acta, 229, 8–2, 2017
- Reversible TAD chemistry as a convenient tool for the design of (re)processable PCL-based shape-memory materials, T. Defize, R. Riva, J.-M. Thomassin, M. Alexandre, N. Van Herck, F.E. Du Prez, C. Jérôm, Macromol. Rapid Comm., 38, 1600517, 2017
- Melamine-formaldehyde microcapsules: micro- and nanostructural characterization with electron microscopy, H. Heidari, G. Rivero, H. Idrissi, D. Ramachandran, S. Cakir, R. Egoavil, M. Kurttepeli, A.C. Crabbé, T. Hauffman, H. Terryn, F.E. Du Prez, D. Schryvers, Microscopy and microanalysis, 22, 1222-1232, 2016
- Quantitative First Principles Kinetic Modeling of the Aza-Michael Addition to Acrylates in Polar Aprotic Solvents, G. Desmet, D. D'hooge, P. Omurtag, P. Espeel, G. Marin, F.E. Du Prez, M-F. Reyniers, J. Org. Chem., 81, 12291-12302, 2016
- Reversible TAD chemistry as a convenient tool for the design of (re)processable PCL-based shape-memory materials, T. Defize, R. Riva, J-M Thomassin, M. Alexandre, N. Van Herck, F.E. Du Prez, C. Jérôm, Macromol. Rapid Comm., accepted, 2016
- Library of thiolactone building blocks with potential in sustainable functional coatings, D. Frank, P. Espeel, S. Claessens, E. Mes, F.E. Du Prez, Tetrahedron, 72, 6616-6625, 2016
- High molecular weight poly(cycloacetals) towards processable polymer materials, S. Lingier, S. Nevejans, P. Espeel, S. De Wildeman, F.E. Du Prez, Polymer, 103, 98-103, 2016
- One-pot automated synthesis of quasi triblock copolymers for self-healing physically crosslinked hydrogels, L. Voorhaar, B. De Meyer, F.E. Du Prez, R. Hoogenboom, Macromol. Rapid Commun., 37, 1682-1688, 2016
- Protected Thiol Strategies in Macromolecular Design, F. Goethals, D. Frank, F. Du Prez, Prog. Polym. Sci. , DOI: 10.1016/j.progpolymsci.2016.09.003, 2016
- Squaric ester amides as hydrolysis-resistant functional groups for protein-conjugation of RAFT-derived polymers, Z. Zhang, N. Vanparijs, S. Vandewalle, F. E. Du Prez, L. Nuhn, B.G. De Geest, Chemcomm., 7, 7242-7248, 2016
- Click reactive microgels as a strategy towards chemically injectable hydrogels, R. Absil, S. Çakir, S. Gabriele, P. Dubois, C. Barner-Kowollik, F.E. Du Prez, L. Mespouille, Polym. Chem., 7, 6752-6760, 2016
- ADMET and TAD Chemistry: A Sustainable Alliance, L. Vlaminck, K. De Bruycker, O. Türünç, F.E. Du Prez, Polym. Chem., 7, 5655 – 5663, 2016
- Automated synthesis of monodisperse oligomers, featuring sequence control and tailored functionalization, S. Martens, J. Van den Begin, A. Madder, F.E. Du Prez, P. Espeel, JACS, 138, 14182-14185, 2016
- The microstructure of capsule containing self-healing materials: A micro-computed tomography study, J. Van Stappen, T. Bultreys, F.A. Gilabert, X.K.D. Hillewaere, D. Garoz Gómez, K. Van Tittelboom, J. Dhaene, N. De Belie, W. Van Paepegem, F.E. Du Prez, V. Cnudde, Mat. Charact., 119, 99-109, 2016
- Thiolactone-based Polymers for Formaldehyde Scavenging Coatings, C. Resetco, D. Frank, T. Dikić, S. Claessens, T. Verbrugge, F.E. Du Prez, Eur. Polym. J., 82, 166–174, 2016
- One-pot Modular Synthesis of Functionalized RAFT agents derived from a single Thiolactone Precursor, S. Martens, F. Driessen, S. Wallyn, O. Türünç, F.E. Du Prez, P. Espeel, ACS Macro Lett., 5, 942−945, 2016
- Macromolecular Coupling in seconds of Triazolinedione End-functionalized Polymers prepared by RAFT Polymerization, S. Vandewalle, S. Billiet, F. Driessen, F.E. Du Prez, ACS Macro Lett., 5, 766-771, 2016
- Simple design of monodisperse chemically crosslinked plant oil nanoparticles by triazolinedione-ene chemistry, S. Chattopadhyay, F.E. Du Prez, Eur. Polym. J., 81, 77–85, 2016
- Double Modification of Polymer End Groups through Thiolactone Chemistry, F. Driessen, S. Martens, B. De Meyer, F.E. Du Prez, P. Espeel, Macromol. Rapid Comm., 37, 947-951, 2016
- Synthesis and evaluation of 9-substituted anthracenes with potential in reversible polymer systems, J. Van Damme, L. Vlaminck, G. Van Assche, B. Van Mele, O. van den Berg, F.E. Du Prez, Tetrahedron, 72, 4303-4311, 2016
- Triazolinediones as highly enabling synthetic tools, K. De Bruycker, S. Billiet, H.A. Houck, S. Chattopadhyay, J.M. Winne, F.E. Du Prez, Chem. Rev., 116, 3919−3974, 2016
- A Biomass Approach toward Robust, Sustainable Multiple Shape-Memory Materials, Z. Wang, Y. Zhang, L. Yuan, J. Hayat, N. Trenor, M. Lamm, L. Vlaminck, S. Billiet, F.E. Du Prez, Z. Wang, C. Tang, ACS Macro Lett., 5, 602-606, 2016
- Comparison of metal free polymer-dye conjugation strategies in protic solvents, J. Gaitzsch, M. Delahaye, A. Poma, F.E. Du Prez, G. Battaglia, Polym. Chem., 7, 3046 - 3055, 2016
- Thiolactone Chemistry and Copper-mediated CRP for the Development of well-defined Amphiphilic Dispersing Agents, F. Driessen, R. Herckens, P. Espeel, F.E. Du Prez, Polym. Chem., 7, 1632-1641, 2016
- Tunable temperature responsive liquid chromatography through thiolactone-based immobilization of poly(N-isopropylacrylamide), A.J. Satti, P. Espeel, S. Martens, T. Van Hoeylandt, F.E. Du Prez, F. Lynen, J. Chrom. A., 1426, 126–132, 2016
- Vitrimers: permanent organic networks with glass-like fluidity, W. Denissen, J.M. Winne, F.E. Du Prez, Chem. Sci., 7, 30-38, 2016
- One-pot double modification of polymers based on thiolacton chemistry, P. Espeel, F.E. Du Prez,Adv. Polym. Sci., 269, 105-132, 2015
- Metal−Organic Frameworks Encapsulated in Photocleavable Capsules for UV-Light Triggered Catalysis, W.P.R. Deleu, G. Rivero, R.F.A. Teixeira, F.E. Du Prez, D.E. De Vos, Chem. Mater., 27, 5495−5502, 2015
- Rewritable polymer brush micropatterns grafted by triazolinedione click chemistry, O. Roling, K. De Bruycker, B. Vonhören, L. Stricker, M. Körsgen, H.F. Arlinghaus, B-J Ravoo, F.E. Du Prez, Angew. Chem. Int. Ed., 54, 1-5, 2015
- Renewable thermoplastic polyurethanes containing rigid spiroacetal moieties, S. Lingier, P. Espeel, S. Suarez Suarez, O. Türünç, S. De Wildeman, F.E. Du Prez, Eur. Polym. J., 70, 232–239, 2015
- The use of triazolinedione click chemistry for tuning the mechanical properties of electrospun SBS-fibers, S. van der Heijden, K. De Bruycker, R. Simal, F.E. Du Prez, K. De Clerck, Macromol., 48, 6474–6481, 2015
- Computational Study and Kinetic Analysis of the Aminolysis of Thiolactones, G. Desmet, D.R. D’hooge, G.B. Marin, F.E. Du Prez, P. Espeel, M.-F. Reyniers, J. Org. Chem, 80, 8520–8529 , 2015
- Poly(thiolactone) Homo- and Copolymers from Maleimide Thiolactone: Synthesis and Functionalization, T. Rudolph, P. Espeel, F.E. Du Prez, F.H. Schacher, Polym. Chem., 6, 4240-4251, 2015
- Sustainable Thermoplastic Elastomers Derived from Plant Oil and Their "Click-Coupling" via TAD Chemistry, Z. Wang, L. Yuan, N.M. Trenor, L. Vlaminck, S. Billiet, A. Sarkar, F.E. Du Prez, M. Stefik, C. Tang, Green Chemistry, 17, 3806-3818, 2015
- Precision Multisegmented Macromolecular Lineups: A Display of Unique Control over Backbone Structure and Functionality, F. Driessen, F.E. Du Prez, P. Espeel, ACS Macro lett., 4, 616−619, 2015
- "Click"-Inspired Chemistry in Macromolecular Science: Matching Recent Progress and User Expectations, P. Espeel, F.E. Du Prez, Macromol., 48, 2-14, 2015
- One-pot multi-step reactions based on thiolactone chemistry: A powerful synthetic tool in polymer science, P. Espeel, F.E. Du Prez, Eur. Polym. J., 62, 247–272, 2015
- Fifteen Chemistries for Autonomous Extrinsic Self-Healing Polymers and Composites, X.K.D. Hillewaere, F.E. Du Prez, Prog. Polym. Sci., 49–50, 121–153, 2015
- Efficient microencapsulation of a liquid isocyanate with in situ shell functionalization, L.-T.T. Nguyen, X.K.D. Hillewaere, R.F.A. Teixeira, O. van den Berg, F.E. Du Prez, Polym. Chem., 6, 1159 – 1170, 2015
- From plant oils to plant foils: Straightforward functionalization and crosslinking of natural plant oils with triazolinediones, O. Türünç, S. Billiet, K. De Bruycker, S. Ouardad, J. Winne, F.E. Du Prez, Eur. Polym. J., 65, 286–297, 2015
- A Shape-Recovery Polymer Coating for the Corrosion Protection of Metallic Surfaces, A. Lutz, O. van den Berg, J. Van Damme, K. Verheyen, E. Bauters, I. De Graeve, F.E. Du Prez, H. Terryn, Appl. Mater. Interfaces, 7, 175-183, 2015
- Vinylogous urethane vitrimers, W. Denissen, G. Rivero, R. Nicolaÿ, L. Leibler, J. Winne, F.E. Du Prez, Adv. Funct. Mat., 25, 2451-2457, 2015
- Ultrafast layer-by-layer assembly of thin organic films based on triazolinedione click chemistry, B. Vonhören, O. Roling, K. De Bruycker, R. Calvo, F.E. Du Prez, BJ Ravoo, ACS Macro Lett.,4, 331−334, 2015
- Thermoresponsive hyperbranched glycopolymers: Synthesis, characterization and lectin interaction studies, S. Vandewalle, S. Wallyn, S. Chattopadhyay, R. Becer, F.E. Du Prez, Eur. Polym. J., 69, 490–498, 2015
- In-depth numerical analysis of the TDCB specimen for characterization of self-healing polymers, D. Garoz Gómez, F.A. Gilabert, E. Tsangouri, D. Van Hemelrijck, X.E. Hillewaere, F.E. Du Prez, W. Van Paepegem, Int. J. Solids Struct., 64–65, 145-154, 2015
- Polythiolactone-Based Redox-Responsive Layers for the Reversible Release of Functional Molecules, S. Belbekhouche, S. Reinicke, P. Espeel, F.E. Du Prez, P. Eloy, C. Dupont-Gillain, A.M. Jonas, S. Demoustier-Champagne, K. Glinel,Appl. Mater. Interfaces, 6, 22457−22466, 2014
- Microencapsulation of Active Ingredients Using PDMS as Shell Material, R.F.A. Teixeira, O. van den Berg, L.-T.T. Nguyen, K. Fehér, F.E. Du Prez, Macromol., 47, 8231-8237, 2014
- Triazolinediones enable ultrafast and reversible click chemistry for the design of dynamic polymer systems, S. Billiet, K. De Bruycker, F. Driessen, H. Goossens, V. Van Speybroeck, J.M. Winne, F.E. Du Prez, Nat. Chem., 6(9), 815-821, 2014
- Synthesis of multi-functionalized hydrogels by a thiolactone-based synthetic protocol, S. Reinicke, P. Espeel, M.M. Stamenović, F.E. Du Prez, Polym. Chem., 5, 5461-5470, 2014
- Collapsing and reswelling kinetics of thermoresponsive polymers on surfaces: a matter of confinement and constraints, N. Willet, S. Gabriel, C. Jérôme, F.E. Du Prez, A-S. Duwez, Soft Matter, 10, 7256-7261, 2014
- Autonomous self-healing of epoxy thermosets with thiol-isocyanate chemistry, X. Hillewaere, R. Teixeira, L.-T.T. Nguyen, J. Ramos, H. Rahier, F.E. Du Prez, Adv. Funct. Mater., 24, 5575–5583, 2014
- Control of glycopolymer nanoparticle morphology by a one-pot, double modification procedure using thiolactones, Y. Chen, P. Espeel, S. Reinicke, F.E. Du Prez, M.H. Stenzel, Macromol Rapid Comm., 35, 1128−1134, 2014
- Cu(0)-mediated polymerization of hydrophobic acrylates using high-throughput experimentation, L. Voorhaar, S. Wallyn, F.E. Du Prez, R. Hoogenboom, Polym. Chem., 5, 4268-4276, 2014
- Deconvolution of overlapping spectral polymer signals in sizeexclusion separation-diode array detection separations byimplementing a multivariate curve resolution method optimized byalternating least , T. Van Hoeylandt, K. Chen, F.E. Du Prez, F. Lynen, J. Chromatogr. A., 1342, 63–69, 2014
- One-pot thermo-remendable shape memory polyurethanes, G. Rivero, L.-T.T. Nguyen, X.K.D. Hillewaere, F.E. Du Prez, Macromol., 47, 2010-2018, 2014
- Preparation of Thiol-Ene Curable, Low Modulus Dry Silicone-Gel Materials, O. van den Berg, L.-T.T. Nguyen, R.F.A. Teixeira, F. Goethals, C. Özdilek, F.E. Du Prez, Macromol., 47, 1292-1300, 2014
- Diversely Substituted Polyamide Structures through Thiol-Ene Polymerization of Renewable Thiolactone Building Blocks, F. Goethals, S. Martens, P. Espeel, O. van den Berg, F.E. Du Prez, Macromol., 47, 61-69, 2014
- Straightforward RAFT-Procedure for the Synthesis of Heterotelechelic Poly(acrylamide)s, S. Wallyn, Z. Zhang, F. Driessen, J. Pietrasik, B.G. De Geest, R. Hoogenboom, F.E. Du Prez, Macromol. Rapid. Comm., 35, 405-411, 2014
- MacroRAFT agents from renewable resources and their use as polymeric scaffolds in a grafting from approach, S. De Smet, S. Lingier, F.E. Du Prez, Polym. Chem, 5, 3163 – 3169, 2014
- Low Modulus Dry Silicone-Gel Materials by Photoinduced Thiol−Ene Chemistry, O. van den Berg,† L-T T. Nguyen, R.F.A. Teixeira, F. Goethals, C. Özdilek, S. Berghmans, F.E. Du Prez†, Macromol., 47, 1292–1300, 2014
- P. Espeel, F. Goethals, F.E. Du Prez, "One-pot Double Modification of Polymers based on Thiolactone Chemistry", in "Advances in Polymer Science"
- P. Espeel, F. Goethals, F.E. Du Prez, ”Thiolactones as Functional Handles for Polymer Synthesis and Modification”, in “Thiol-X chemistries in Polymer and Materials Science”, RSC publishing, ed. Andrew Lowe and Christopher Bowman, 2013 (ISBN: 978-1-84973-660-2)
- F.E. Du Prez, E. Goethals, R. Hoogenboom, “Cationic polymerizations” in Handbook of Polymer Synthesis, Characterization, and Processing, First Edition. Edited by Enrique Saldivar-Guerra and Eduardo Vivaldo-Lima. John Wiley & Sons, Inc., 2013 (ISBN: 978-0-470-63032-7)
- R.F.A. Teixeira, X.K.D. Hillewaere, S. Billiet, F.E. Du Prez, “Chemistry of crosslinking processes for self-healing polymers”, in “self-healing polymers: from principles to applications”, Wiley VCH, ed. Wolfgang Binder, 2013 (ISBN: 978-3-527-33439-1)
- E.J. Goethals, B. Dervaux (2012) ROP of Cyclic Amines and Sulfides. In: Matyjaszewski K and Möller M (eds.) Polymer Science: A Comprehensive Reference, Vol 4, pp. 309–330. Amsterdam: Elsevier BV
- T.J.A. Loontjens, E.J. Goethals (2012) Chain Extension by Ring Opening. In: Matyjaszewski K and Möller M (eds.) Polymer Science: A Comprehensive Reference, Vol 4, pp. 633–644. Amsterdam: Elsevier BV
- C. Eisenbach, N. Bulychev, K. Dirnberger, B. Dervaux, F. Du Prez, V. Zubov, "Structure Formation of Adsorption Layers of Ionic-Amphiphilic Copolymers on Inorganic and Organic Pigment Surfaces as studied by ESA" in "Amphiphiles: Molecular Assembly and applications", ACS Symposium Series 1070, Ed. Ramanathan Nagarajan, 2011 (ISBN 978-0-8412-2650-0)
- W. Van Camp, B. Dervaux, M. Lammens, L. Van Renterghem, F.E. Du Prez, “From Novel block-like copolymers to reactive nanoparticles: ATRP and “click” chemistry” as synthetic tools” (chapter 7) in NATO-book, Ed. E. Khosravi, “New Smart Materials via metal mediated macromolecular engineering”, Springer, 2009 (ISBN 978-90-481-3276-8)
- D. Fournier, L. Billiet, F.E. Du Prez, “Click chemistry and step-growth polymerization: the ideal combination for the rejuvenation of industrial polymers” (chapter 9) in NATO-book, Ed. E. Khosravi, “New Smart Materials via metal mediated macromolecular engineering”, Springer, 2009 (ISBN 978-90-481-3276-8)
- M. Lammens, F.E. Du Prez, Highly branched functional polymer architectures by click chemistry strategies
in "Complex macromolecular Architectures: Synthesis, characterization and self-assembly",
Ed. Filip Du Prez, Nikos Hadjichristidis, Yasuyuki Tezuka and Akira Hirao, Wiley-VCH, Weinheim Germany, 2011, (ISBN 978-0-470-82513-6)
- W. Van Camp, F.E. Du Prez, “Controlled Radical Polymerization of
1-Ethoxyethyl (Meth)acrylate: Novel Route for the Synthesis of Poly((meth)acrylic acid) Containing Polymer Structures” (Chapter 13)
in “ACS Symposium Book”, volume 944, Ed. K. Matyjaszewski, "Controlled/Living Radical
Polymerization: From Synthesis to Materials", Wiley VCH, 2006, (ISBN 0-8412-3991-3)
- W. Loos, F.E. Du Prez, “The sol-gel approach towards thermo-responsive poly(N-isopropyl acrylamide) hydrogels with improved mechanical properties”
in “Macromolecular Symposia, Vol. 210, Reactive Polymers”, H. Adler, ed., Wiley VCH, 2004 (ISBN 3-527-31043-6)
- W. Reyntjens, L. Jonckheere, E. Goethals and F. Du Prez, "Thermosensitive polymer structures based on segmented copolymer networks"
in “Macromolecular Symposia, Vol. 164, Reactive Polymers”, H. Adler, ed., Wiley VCH, 2003 (ISBN 3-527-30326-x)
- N. A. Yanul, Y.E. Kirsch, S. Verbrugghe, E.J. Goethals and F.E. Du Prez, “Synthesis and thermo-responsive properties of poly(N-vinyl caprolactam) / polyether segmented networks”
in “Tailored Polymers & Applications”, Y. Yagci, M.K. Mishra, O. Nuyken, K. Ito, G. Wnek, eds., VSH Publishers, pp. 139-149, 2001 (ISBN 90-6764-326-2)
- F.E. Du Prez, D. Christova & E.J. Goethals, "Amphiphilic multicomponent polymer networks: design, evaluation and applications"
in “the Wiley Polymer Networks Group Review Series”, vol. 2, B.T. Stokke, A. Elgsaeter, Eds., John Wiley & Sons, West Sussex, UK, pp. 255-270, 2000 (ISBN 0-471-98713-1).
- F.E. Du Prez & E.J. Goethals, “Segmented polymer networks by cationic polymerization: design and applications”
in “Cationic Polymerization and Related Processes”, J.E. Puskas, Ed., NATO-ASI Series, Series E, Kluwer Academic Publisher, Dordrecht, Nederland, pp. 75-98, 1999 (ISBN 0-7923-5811-2).
- F.E. Du Prez, P. Tan & E.J. Goethals, "PEO/PMMA (Semi-)interpenetrating Polymer Networks: Synthesis and Phase Diagrams"
in “IPNs around the World: Science and engineering”, S.C. Kim en L.H. Sperling, eds., John Wiley & Sons, West Sussex, UK, Chapter 6, pp. 123-137, 1997 (ISBN 0-471-97077-8).
- EP 19211548.3: “Epoxy-derived covalent adaptable networks and methods of their production” – Inventors: J. Winne, Y. Spiesschaert, C. Taplan, F. Du Prez – Filling date: 26 November 2019
- EP 19203478.3: “A polymer comprising a unit having a tertiary amine and a pendant carboxyl group, a precursor for such polymer and a method to prepare such polymer or such precursor- Inventors: J. Winne, M. Delahaye, F. Du Prez – Filling date: 16 October 2019
- WO2019175346A1: “Indole-based compounds” – Inventors: L. Imbernon, J. De Geeter, F. Du Prez, N. Haucourt, D. Laplace, G. Snellings, P. Van Tittelboom, J. Winne – Priority date: 14 March 2018
- CN108473680A; EP3344682A1; WO2017036525A1; US2018258201A1: “Thiol-acrylate based foam precursor composition” – Inventors: F. Du Prez, O. Turunc – Priority date: 2 September 2015
- EP3233978A1; WO2016097169A1; US2017327625A1; US10246548B2: “Compositions comprising a polymeric network” – Inventors: F. Du Prez, W. Denissen, J. Winne, L. Leibler, R. Nicolay – Priority date: 19 December 2014
- EP3030559A1; EP3030559B1; WO2015018928A1; US2016200707A1; US9586935B2: “Urazole compounds” – Inventors: S. Billiet, J. Winne, F. Du Prez – Priority Date: 9 August 2013
- EP2841490A2; WO13160252A2; US20130287345A1; US8967888B2: “Dry silicone gels and their methods of making them by using thiol-ene chemistry” – Inventors: O. Van den Berg, S. Berghmans, F. Du Prez – Priority Date: 25 April 2012
- EP2522684A1; EP2522685A2; EP2522685A3; US2012289621A1; US8901180B2: “Method for functionalising a thermoset isocyanate-based polymeric solid material” – Inventors: L. Jonckeere, F. Du Prez, T. Nguyen – Priority date: 12 May 2011
- BR112013028982A2; CN103687884A; CN103687884B; EP2707409A2; EP2707409B1; ES2534975T3; KR20140039219A; KR101919176B1; SG194760A1; WO2012154393A2; WO2012154393A3; US2014058037A1; US9644097B2: Stabilizer polymerization process and process for making polymer polyols – Inventors: F. Du Prez, L. Petton, F. Van Damme, J.P. Masy, E. Mes, H. R. Van der Wal, S. Verbrugghe – Priority date: 12 May 2011
- EP2697286A2; WO2012140194A2; WO2012140194A3; US2014039081A1: “Sulphur-containing thermoplastic polymers” – Inventors: F. Du Prez, O. Vandenbergh, S. Verbrugghe– Priority date: 14 April 2011
- AU2009295886A1; AU2016200258A1; AU2016200258B2; BRPI0913683A2; CA2738602A1; CN102369105A; EA201100533A1; EP2352641A1; EP3508342A1; WO2010034776A1; JP2012503473A; KR20110098996A; KR20170017001A; KR101764592B1; MX2011003048A; MX348019B; US2011217758A1: “Incorporation of thermo-resistant and/or pressure-resistant organisms in materials” – Inventors: J. Mertens, L. Beladjal, F. Devlieghere, S. Verbrugghe, F. Du Prez, T. Anthierens, A. Ouchen – Priority date: 24 September 2008
- EP2009039A1; EP2164888A2; EP2164888B1; WO2009000892A2; WO2009000892A3; ES2396174T3; US2010234482A1; US8889799B2: “Functionalised polyurethanes” – Inventors: F. Du Prez, D. Fournier – Priority date: 27 June 2007
- EP2090592A1: “Biodegradable gels based on click chemistry” – Inventors: B. De Geest, F Du Prez, W. E. Hennink, W. Van Camp – Priority date: 31 July 2007
- WO2006002496A1; EP1776399A1; EP1776399B1; JP2008504411A; US2013096268A1; US2008262160A1: “Monodisperse polymers containing (alkyl)acrylic acid moieties, precursors and methods for making them and their applications” – Inventors: F. Du Prez, W. Van Camp, S. Bon – Priority date: 1 July 2004
Some PhD-theses are available on the website of the UGent Library.
(D = Dutch, E = English):
- Yann Spiesschaert: "Vinylogous urethane vitrimers - Towards a closed recycling loop for thermoset materials" (E) (2020)
- Stef Vandewalle: "Macromolecular functionalization and coupling : from permanent to dynamic linking" (E) (2020)
- Maarten Delahaye: "Recyclable polymer networks based on internally catalysed exchange reactions" (E) (2020)
- Niels Van Herck: "Towards sustainable dynamic polymer materials - from self-healing to reprocessability" (E) (2020)
- Maarten Delahaye: "Recyclable polymer networks based on internally catalysed exchange reactions" (E) (2020)
- Joshua Holloway: "From sequence-defined macromolecules to macromolecular pin codes" (E) (2019)
- Steven Martens: "Amine-thiolactone-ene conjugation : from polymer modification to sequence-controlled polymers" (E) (2019)
- Laetitia Vlaminck: "Triazolinedione building blocks: their sustainable synthesis and macromolecular implementation" (E) (2019)
- Daniel Frank: "Thiolactone derivatives : from bio-based building blocks towards sustainable materials" (E) (2018)
- Benjamin Hendriks: "Self-crosslinking resins and vitrimers based on thiol-ene chemistry" (E) (2018)
- Sophie Lingier: "Polycycloacetals from renewable resources" (E) (2017)
- Kevin De Bruycker: "Where small molecules and polymer materials meet: triazolinedione chemistry at the interface" (E) (2017)
- Cristina Resetco: "Multifunctional polymer materials based on sustainable thiolactone building blocks" (E) (2017)
- Remi Absil: "From microparticles to injectable hydrogels : the role of click coupling" (E) (2017)
- Frank Driessen: "Functional and amphiphilic copolymers by means of copper-mediated polymerization" (E) (2017)
- Wim Denissen: "Vitrimers based on vinylogous acyl exchange reactions" (E) (2017)
- Stijn Billiet: "Triazolinediones for the development of a versatile click chemistry platform applied in polymer science" (E) (2016)
- Sanne De Smet: "Synthesis of segmented polymer structures based on renewable resources" (E) (2016)
- Xander Hillewaere: "Thiol-based chemistries for extrinsic self-healing thermosets" (E) (2015)
- Sofie Wallyn: "Versatile synthesis of functional hyperbranched polymers by combination of CRP and Thiol-X chemistry" (E) (2015)
- Fabienne Goethals: "Multistep reactions based on thiolactones for the synthesis of functionalized polymers" (E) (2015)
- Michel De Keersmaecker: "The development of an environmentally friendly coating for the corrosion inhibition of lead objects" (E) (2015)
- Milan Stamenovic: "Complex polymer architectures by combination of RAFT and post-polymerization methods" (E) (2013)
- Lionel Petton: "Design and Evaluation of Novel Types of Polymeric Dispersants" (E) (2012)
- Tuğba Dişpinar: "Cryogels and polymeric microcapsules with triggered degradation and release" (E) (2012)
- M. Talha Gokmen: "Complex Polymer Particles via Microfluidics" (E) (2011).
- Leen Billiet: "Functionalization of step-growth polymers by click chemistries" (E) (2011)
- Mieke Lammens: "Highly Functionalized Star-shaped Polymer Structures
via Click Chemistry Design" (E) (2011).
- Bart Dervaux: "Gradient versus block copolymers via atom transfer radical polymerization: continuous preparation via column reactors and comparison of structure property relations en vergelijking van structuur-eigenschapsrelaties" (E) (2010).
- Lies Bonami: "New types of metal complexing polymer structures based on polyamines: synthesis and evaluation" (E) (2010).
- Ondine Confortini: "Thermo-responsive graft copolymers based on poly(methyl vinyl ether): from synthesis to evaluation" (E) (2009).
- Els Eeckhout: "Synthesis and evaluation of supramolecular polymer structures based on cyclodextrine containing polyrotaxanes" (D) (2008).
- Wim Van Camp: " Novel Routes for the Design of Poly((meth)acrylic acid) Containing Polymer Structures by Controlled Radical Polymerization" (E) (2007).
- Lieven Van Renterghem: "Star-shaped polymer structures with reactive end groups for the preparation of a new type of nanoparticles" (D) (2007).
- Jan Heijl: “Fast stimuli-responsive polymer materials from ultrathin hydrogels" (D+E) (2006).
- Katrien Bernaerts: “Advanced polymer architectures with stimuli-responsive properties starting from heterofunctional initiators” (2006).
- Laura Jonckheere: "Development of tailor-made, crosslinked polymer structures as supports of organometal complexes". (D) (2005)
- Wouter Lequieu: "Segmented polymer networks and modified track etched membranes: new materials for thermo-controllable membrane processes". (D) (2005)
- Wouter Loos: "Design of thermo-responsive hybrid hydrogels via the sol-gel process". (D) (2005)
- Beatrice Verdonck: "Design of thermo-responsive polymer surfactants based on poly(methyl vinyl ether)". (D) (2004)
- Kris Vidts: "Design of block copolymer structures for the control of physico-chemical properties of nanodispersions". (D) (2004)
- Peggy Van De Velde: "Development and evaluation of new polymer architectures as pervaporation membranes". (2004)
- Filip Vanhaecke: "Smelt-functionalisatie van isotactisch polypropyleer met cyclische onverzadigde dicarbonzuuranhydrides". (D)(2003)
- Sam Verbrugghe: "Development of ‘intelligent' polymer architectures based on poly(N-vinyl caprolactam)". (D)(2003)
- Kathelijne Cosaert: “Evaluation of positron annihilation life time spectroscopy for the research towards free volume properties of polymer materials” (D)(2003)